Coupling Constants J spinspin coupling scalar coupling or





![Coupling Constants (J) Second-Order Effects ([AX]2) Coupling Constants (J) Second-Order Effects ([AX]2)](https://slidetodoc.com/presentation_image_h/2e4706474aa8d04614f553e27c6819f4/image-28.jpg)

- Slides: 29

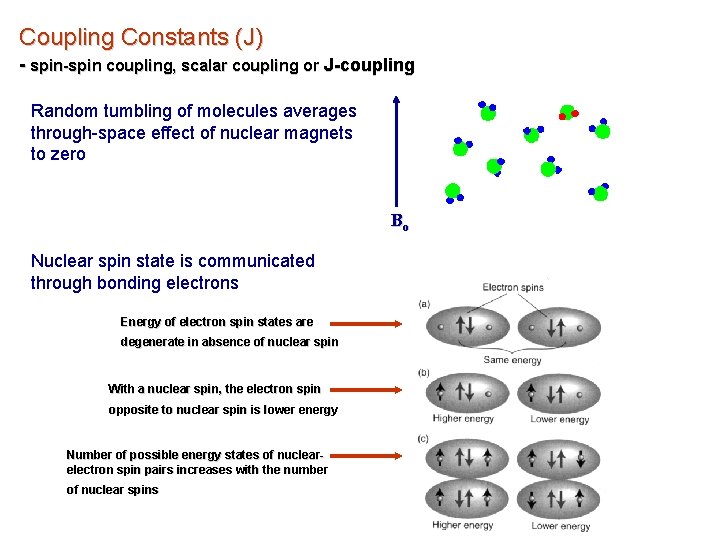
Coupling Constants (J) - spin-spin coupling, scalar coupling or J-coupling Random tumbling of molecules averages through-space effect of nuclear magnets to zero Bo Nuclear spin state is communicated through bonding electrons Energy of electron spin states are degenerate in absence of nuclear spin With a nuclear spin, the electron spin opposite to nuclear spin is lower energy Number of possible energy states of nuclearelectron spin pairs increases with the number of nuclear spins

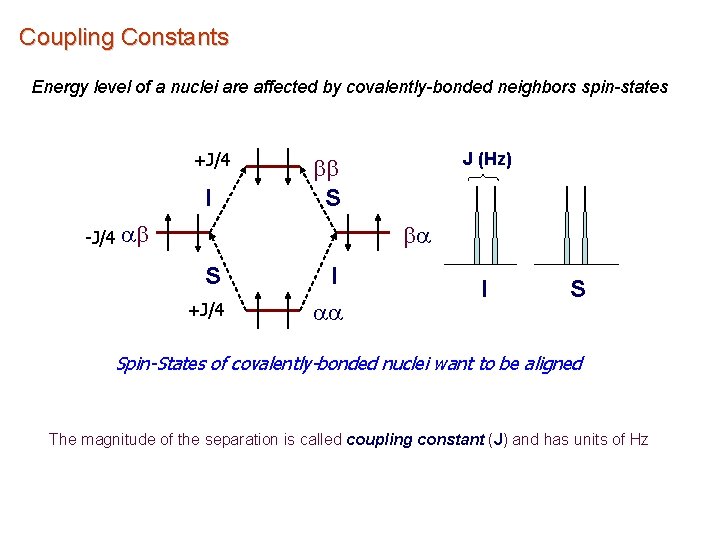
Coupling Constants Energy level of a nuclei are affected by covalently-bonded neighbors spin-states +J/4 I J (Hz) bb S -J/4 ab ba S +J/4 I aa I S Spin-States of covalently-bonded nuclei want to be aligned The magnitude of the separation is called coupling constant (J) and has units of Hz

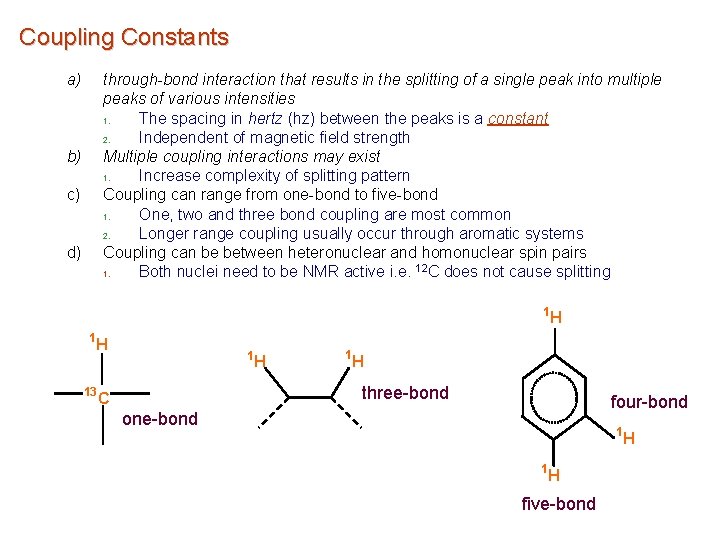
Coupling Constants a) through-bond interaction that results in the splitting of a single peak into multiple peaks of various intensities 1. The spacing in hertz (hz) between the peaks is a constant 2. Independent of magnetic field strength Multiple coupling interactions may exist 1. Increase complexity of splitting pattern Coupling can range from one-bond to five-bond 1. One, two and three bond coupling are most common 2. Longer range coupling usually occur through aromatic systems Coupling can be between heteronuclear and homonuclear spin pairs 1. Both nuclei need to be NMR active i. e. 12 C does not cause splitting b) c) d) 1 1 H 13 1 H H three-bond C four-bond one-bond 1 1 H five-bond H

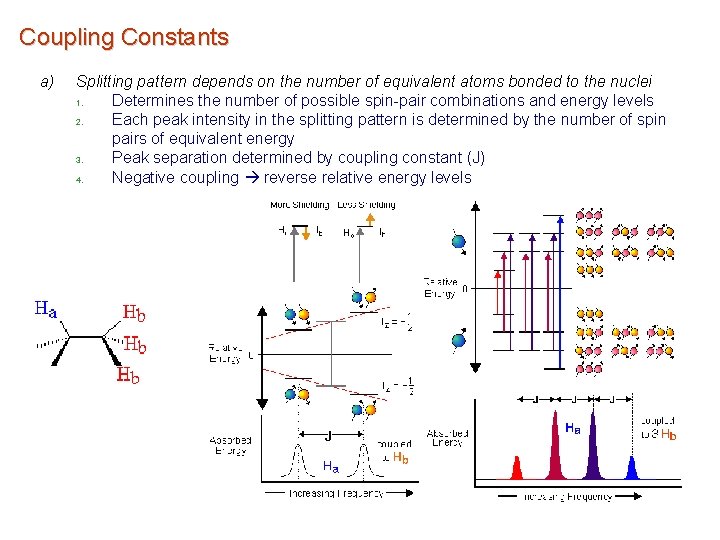
Coupling Constants a) Splitting pattern depends on the number of equivalent atoms bonded to the nuclei 1. Determines the number of possible spin-pair combinations and energy levels 2. Each peak intensity in the splitting pattern is determined by the number of spin pairs of equivalent energy 3. Peak separation determined by coupling constant (J) 4. Negative coupling reverse relative energy levels

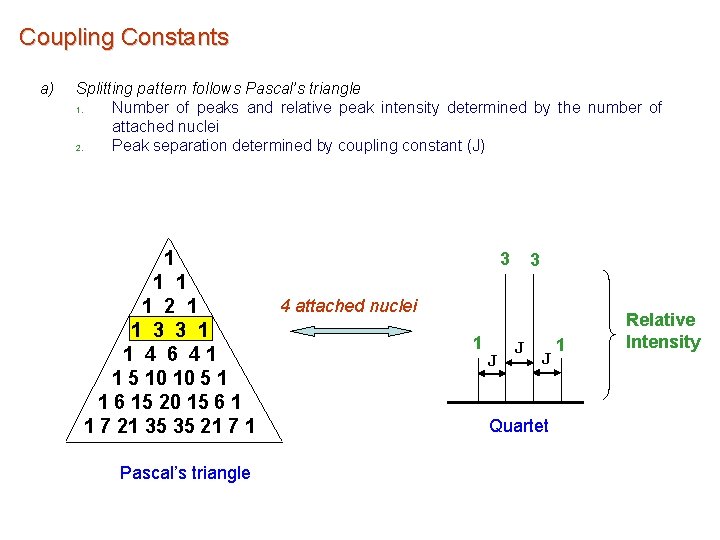
Coupling Constants a) Splitting pattern follows Pascal’s triangle 1. Number of peaks and relative peak intensity determined by the number of attached nuclei 2. Peak separation determined by coupling constant (J) 1 1 2 1 1 3 3 1 1 4 6 41 1 5 10 10 5 1 1 6 15 20 15 6 1 1 7 21 35 35 21 7 1 Pascal’s triangle 3 3 4 attached nuclei 1 J J J Quartet 1 Relative Intensity

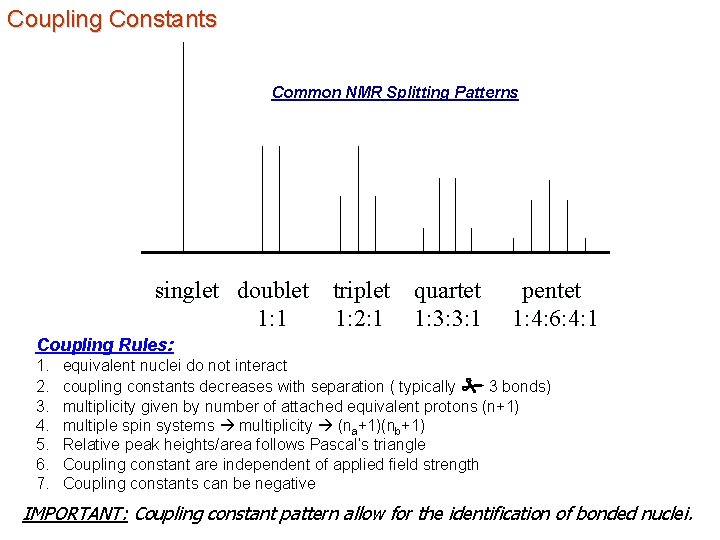
Coupling Constants Common NMR Splitting Patterns singlet doublet 1: 1 triplet quartet 1: 2: 1 1: 3: 3: 1 pentet 1: 4: 6: 4: 1 Coupling Rules: 1. 2. 3. 4. 5. 6. 7. equivalent nuclei do not interact coupling constants decreases with separation ( typically # 3 bonds) multiplicity given by number of attached equivalent protons (n+1) multiple spin systems multiplicity (na+1)(nb+1) Relative peak heights/area follows Pascal’s triangle Coupling constant are independent of applied field strength Coupling constants can be negative IMPORTANT: Coupling constant pattern allow for the identification of bonded nuclei.

Coupling Constants Common NMR Splitting Patterns

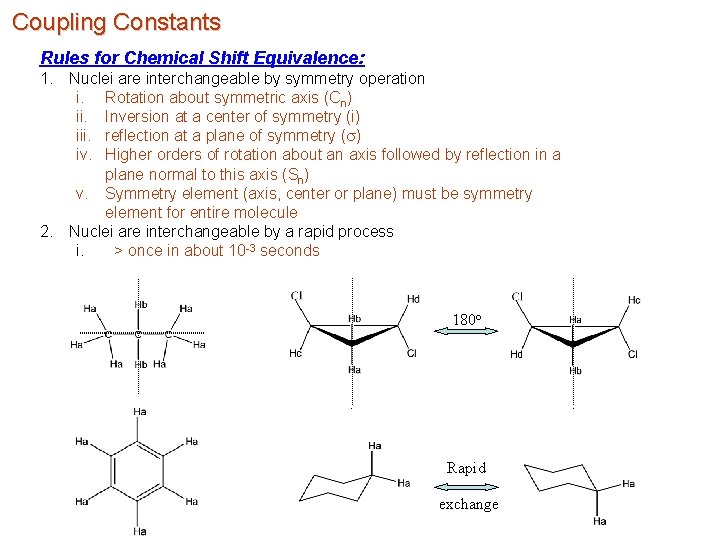
Coupling Constants Rules for Chemical Shift Equivalence: 1. Nuclei are interchangeable by symmetry operation i. Rotation about symmetric axis (Cn) ii. Inversion at a center of symmetry (i) iii. reflection at a plane of symmetry (s) iv. Higher orders of rotation about an axis followed by reflection in a plane normal to this axis (Sn) v. Symmetry element (axis, center or plane) must be symmetry element for entire molecule 2. Nuclei are interchangeable by a rapid process i. > once in about 10 -3 seconds 180 o Rapid exchange

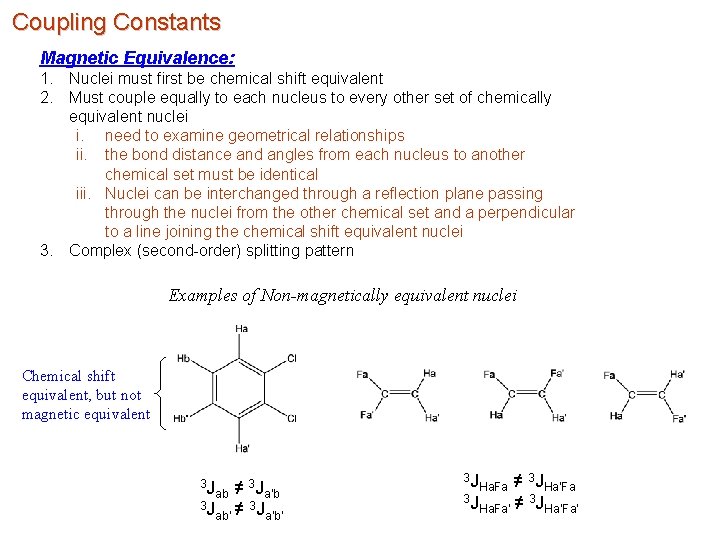
Coupling Constants Magnetic Equivalence: 1. Nuclei must first be chemical shift equivalent 2. Must couple equally to each nucleus to every other set of chemically equivalent nuclei i. need to examine geometrical relationships ii. the bond distance and angles from each nucleus to another chemical set must be identical iii. Nuclei can be interchanged through a reflection plane passing through the nuclei from the other chemical set and a perpendicular to a line joining the chemical shift equivalent nuclei 3. Complex (second-order) splitting pattern Examples of Non-magnetically equivalent nuclei Chemical shift equivalent, but not magnetic equivalent 3 J 3 ab ≠ Ja’b 3 J 3 ab’ ≠ Ja’b’ 3 J 3 Ha. Fa ≠ JHa’Fa 3 J 3 Ha. Fa’ ≠ JHa’Fa’

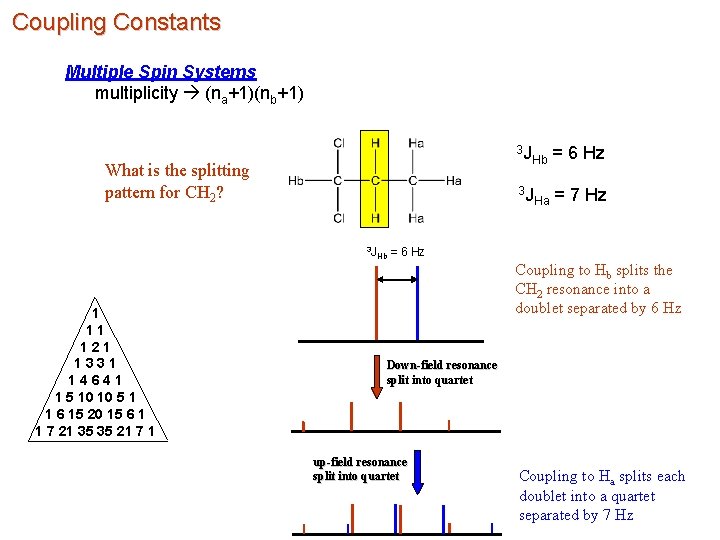
Coupling Constants Multiple Spin Systems multiplicity (na+1)(nb+1) What is the splitting pattern for CH 2? 3 J Hb 1 11 121 1331 14641 1 5 10 10 5 1 1 6 15 20 15 6 1 1 7 21 35 35 21 7 1 3 J Hb = 6 Hz 3 J Ha = 7 Hz = 6 Hz Coupling to Hb splits the CH 2 resonance into a doublet separated by 6 Hz Down-field resonance split into quartet up-field resonance split into quartet Coupling to Ha splits each doublet into a quartet separated by 7 Hz

Coupling Constants What Happens to Splitting Pattern if J changes? 3 J Hb = 7 Hz 3 J Ha = 7 Hz Looks like a pentet! Intensities don’t follow Pascal’s triangle (1 4 6 4 1) 3 J Hb = 6 Hz 3 J Ha = 3 Hz Looks like a sextet! Intensities don’t follow Pascal’s triangle (1 5 10 10 5 1) Occurs because of overlap of peaks within the splitting pattern

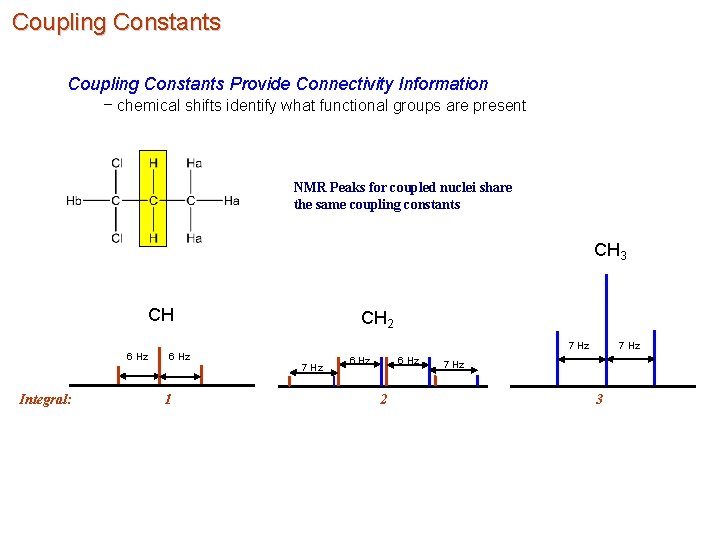
Coupling Constants Provide Connectivity Information – chemical shifts identify what functional groups are present NMR Peaks for coupled nuclei share the same coupling constants CH 3 CH CH 2 7 Hz 6 Hz Integral: 6 Hz 1 7 Hz 6 Hz 2 7 Hz 3

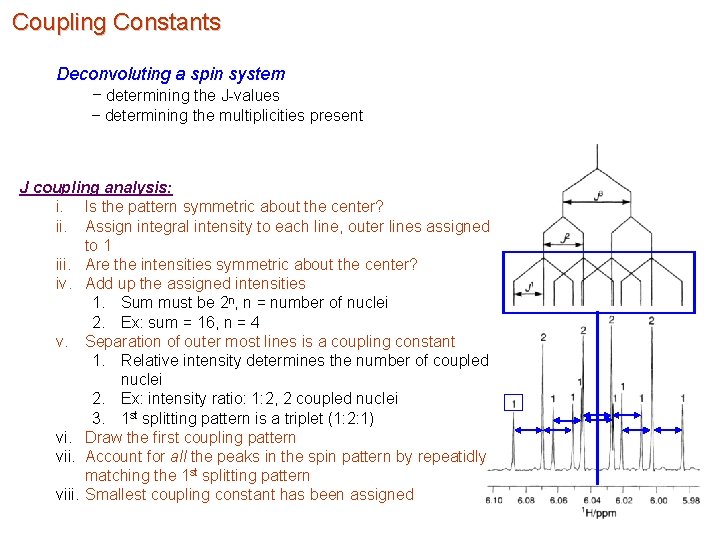
Coupling Constants Deconvoluting a spin system – determining the J-values – determining the multiplicities present J coupling analysis: i. Is the pattern symmetric about the center? ii. Assign integral intensity to each line, outer lines assigned to 1 iii. Are the intensities symmetric about the center? iv. Add up the assigned intensities 1. Sum must be 2 n, n = number of nuclei 2. Ex: sum = 16, n = 4 v. Separation of outer most lines is a coupling constant 1. Relative intensity determines the number of coupled nuclei 2. Ex: intensity ratio: 1: 2, 2 coupled nuclei 3. 1 st splitting pattern is a triplet (1: 2: 1) vi. Draw the first coupling pattern vii. Account for all the peaks in the spin pattern by repeatidly matching the 1 st splitting pattern viii. Smallest coupling constant has been assigned

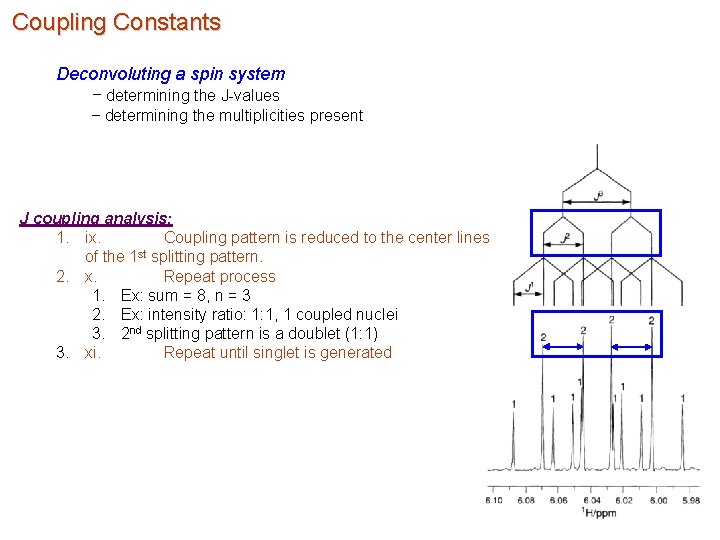
Coupling Constants Deconvoluting a spin system – determining the J-values – determining the multiplicities present J coupling analysis: 1. ix. Coupling pattern is reduced to the center lines of the 1 st splitting pattern. 2. x. Repeat process 1. Ex: sum = 8, n = 3 2. Ex: intensity ratio: 1: 1, 1 coupled nuclei 3. 2 nd splitting pattern is a doublet (1: 1) 3. xi. Repeat until singlet is generated

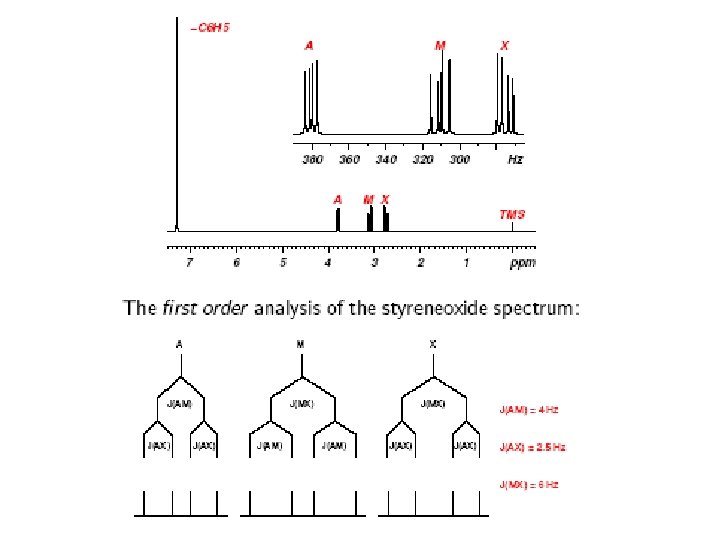
Coupling Constants Demo ACD HNMR Viewer software – first order coupling constants

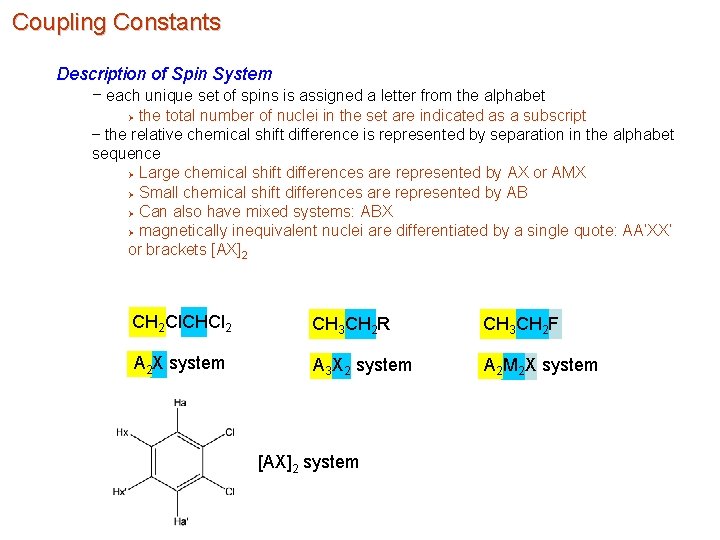
Coupling Constants Description of Spin System – each unique set of spins is assigned a letter from the alphabet the total number of nuclei in the set are indicated as a subscript – the relative chemical shift difference is represented by separation in the alphabet sequence Ø Large chemical shift differences are represented by AX or AMX Ø Small chemical shift differences are represented by AB Ø Can also have mixed systems: ABX Ø magnetically inequivalent nuclei are differentiated by a single quote: AA’XX’ or brackets [AX]2 Ø CH 2 Cl. CHCl 2 CH 3 CH 2 R CH 3 CH 2 F A 2 X system A 3 X 2 system A 2 M 2 X system [AX]2 system


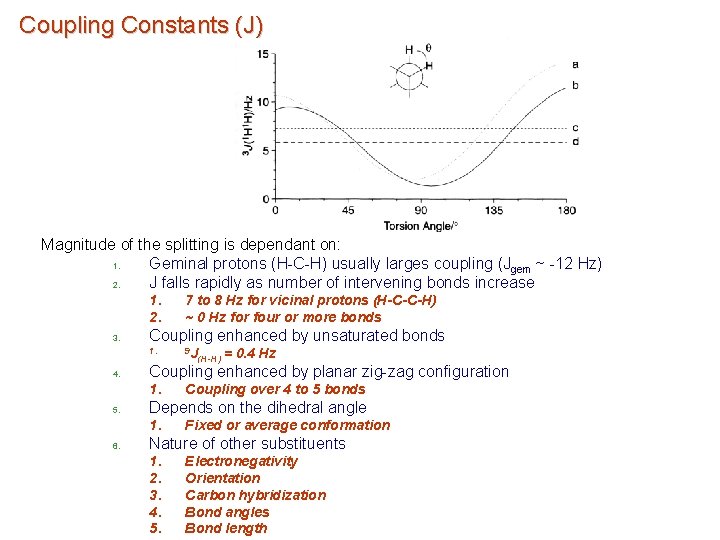
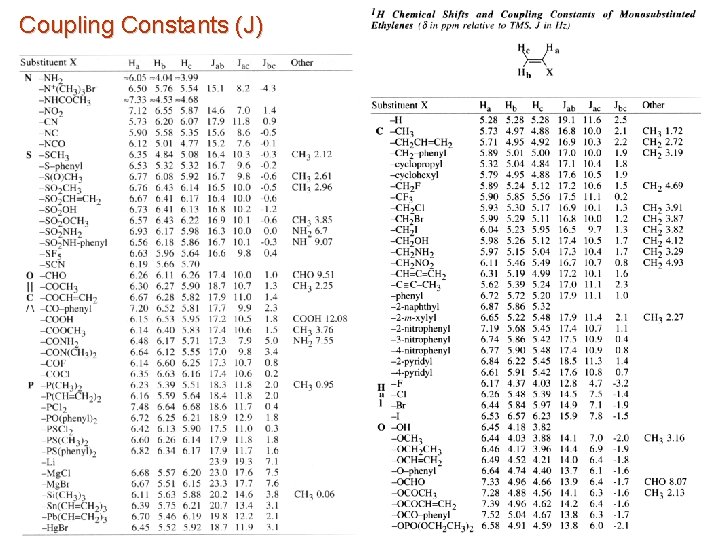
Coupling Constants (J) Observed splitting is a result of this electron-nucleus hyperfine interaction a) Magnitude of the splitting is dependant on: 1. Number of bonds 2. Bond order (single, double triple) 3. Angles between bonds 4. Proportional to gagb 5. s character of bonding orbital 1. 6. Attenuated as the number of bonds increase – b) Increases periodically with atomic number (see chemical shift range) not usually seen over more than 5 to 6 bonds Coupling is measured in hertz (Hz) 1. Range from 0. 05 Hz to thousands of Hz 2. Can be negative sign

Coupling Constants (J) Magnitude of the splitting is dependant on: 1. Geminal protons (H-C-H) usually larges coupling (Jgem ~ -12 Hz) 2. J falls rapidly as number of intervening bonds increase 1. 2. 3. Coupling enhanced by unsaturated bonds 1. 4. (H-H) = 0. 4 Hz Coupling over 4 to 5 bonds Depends on the dihedral angle 1. 6. 9 J Coupling enhanced by planar zig-zag configuration 1. 5. 7 to 8 Hz for vicinal protons (H-C-C-H) ~ 0 Hz for four or more bonds Fixed or average conformation Nature of other substituents 1. 2. 3. 4. 5. Electronegativity Orientation Carbon hybridization Bond angles Bond length

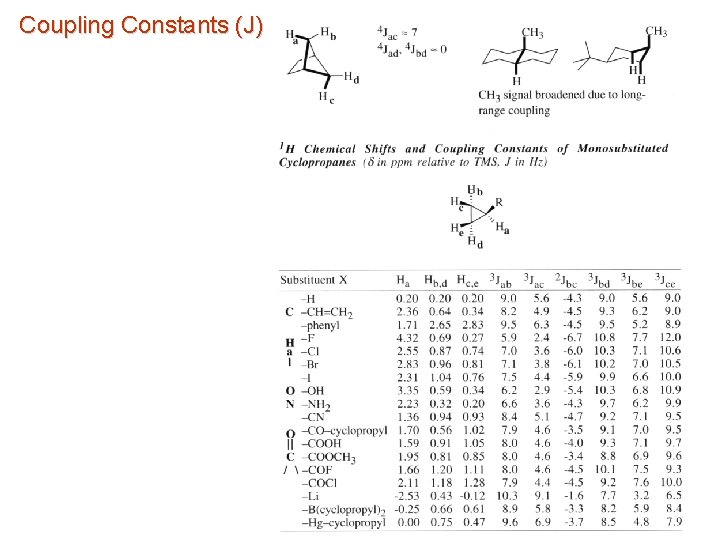
Coupling Constants (J)

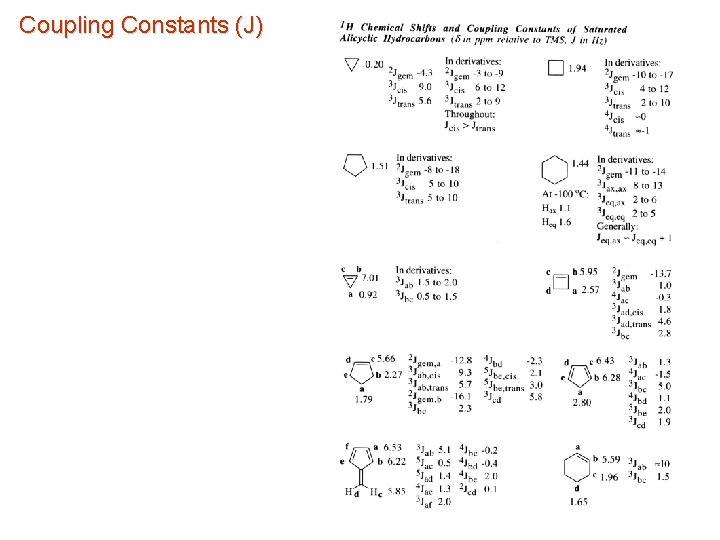
Coupling Constants (J)

Coupling Constants (J)

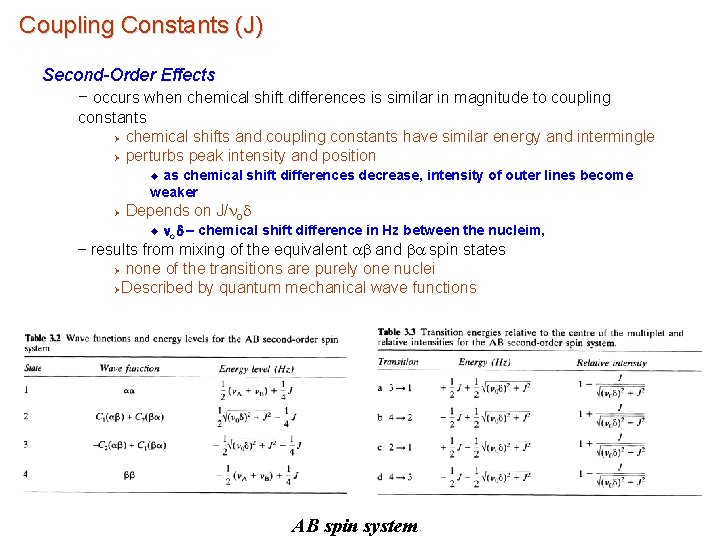
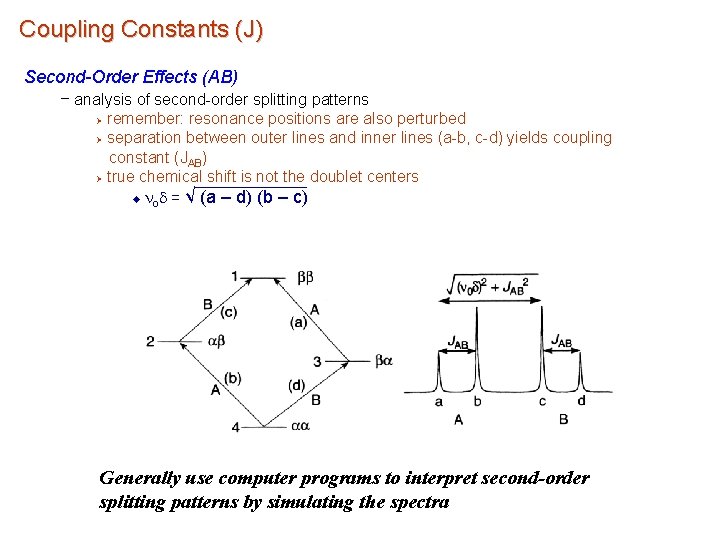
Coupling Constants (J) Second-Order Effects – occurs when chemical shift differences is similar in magnitude to coupling constants Ø chemical shifts and coupling constants have similar energy and intermingle Ø perturbs peak intensity and position as chemical shift differences decrease, intensity of outer lines become weaker u Ø Depends on J/nod u nod – chemical shift difference in Hz between the nucleim, – results from mixing of the equivalent ab and ba spin states Ø none of the transitions are purely one nuclei ØDescribed by quantum mechanical wave functions AB spin system

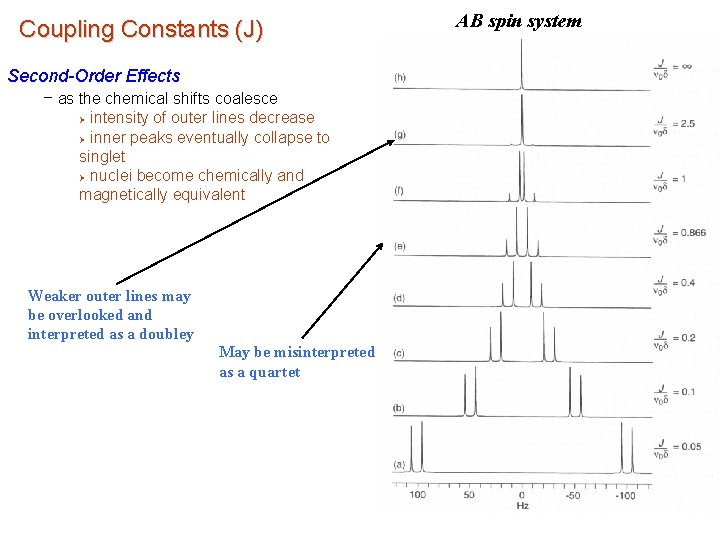
Coupling Constants (J) Second-Order Effects – as the chemical shifts coalesce intensity of outer lines decrease Ø inner peaks eventually collapse to singlet Ø nuclei become chemically and magnetically equivalent Ø Weaker outer lines may be overlooked and interpreted as a doubley May be misinterpreted as a quartet AB spin system

Coupling Constants (J) Second-Order Effects (AB) – analysis of second-order splitting patterns remember: resonance positions are also perturbed Ø separation between outer lines and inner lines (a-b, c-d) yields coupling constant (JAB) Ø true chemical shift is not the doublet centers u nod = √ (a – d) (b – c) Ø Generally use computer programs to interpret second-order splitting patterns by simulating the spectra

Coupling Constants (J) Second-Order Effects (AB 2)

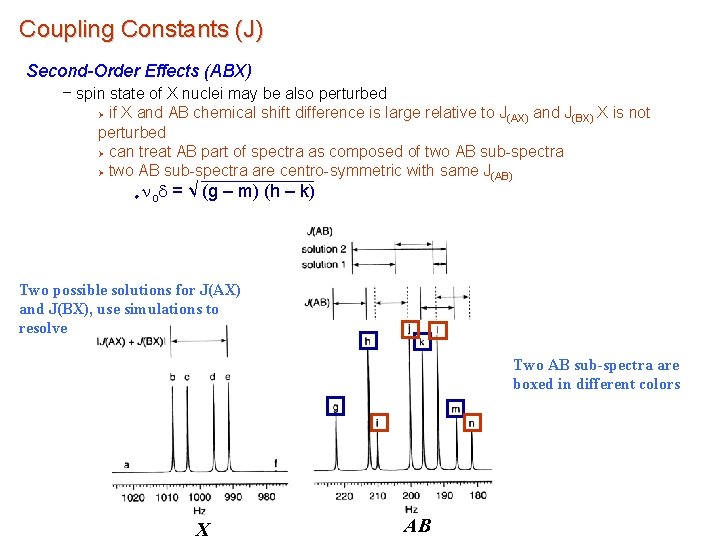
Coupling Constants (J) Second-Order Effects (ABX) – spin state of X nuclei may be also perturbed if X and AB chemical shift difference is large relative to J(AX) and J(BX) X is not perturbed Ø can treat AB part of spectra as composed of two AB sub-spectra Ø two AB sub-spectra are centro-symmetric with same J(AB) Ø u nod = √ (g – m) (h – k) Two possible solutions for J(AX) and J(BX), use simulations to resolve Two AB sub-spectra are boxed in different colors X AB
![Coupling Constants J SecondOrder Effects AX2 Coupling Constants (J) Second-Order Effects ([AX]2)](https://slidetodoc.com/presentation_image_h/2e4706474aa8d04614f553e27c6819f4/image-28.jpg)
Coupling Constants (J) Second-Order Effects ([AX]2)

Coupling Constants (J) Demo ACD HNMR Viewer software – second order coupling constants