ELECTRON CONFIGURATIONS CHEMISTRY 332 WHAT IS AN ELECTRON



- Slides: 19

ELECTRON CONFIGURATIONS CHEMISTRY 332

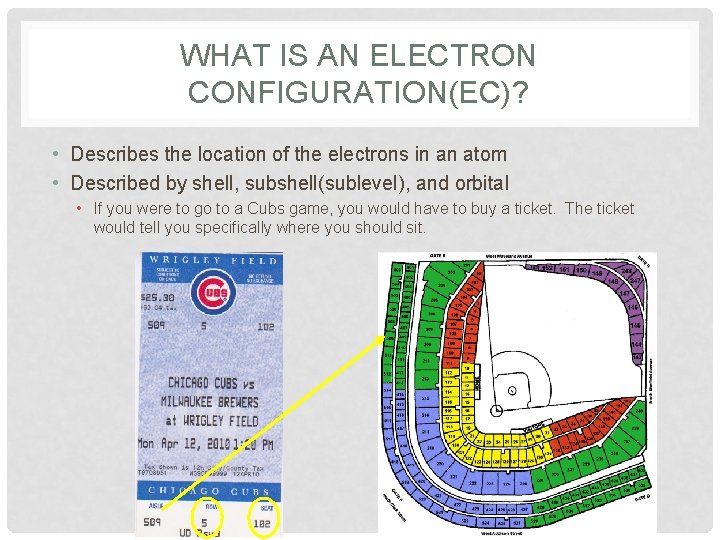
WHAT IS AN ELECTRON CONFIGURATION(EC)? • Describes the location of the electrons in an atom • Described by shell, subshell(sublevel), and orbital • If you were to go to a Cubs game, you would have to buy a ticket. The ticket would tell you specifically where you should sit.

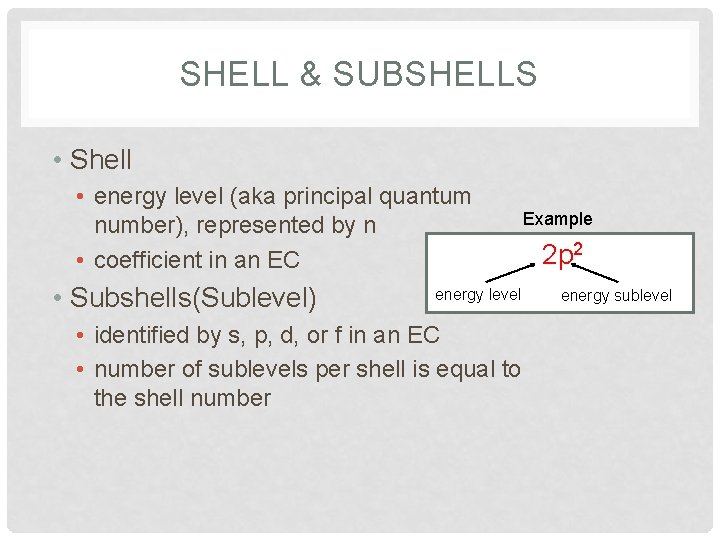
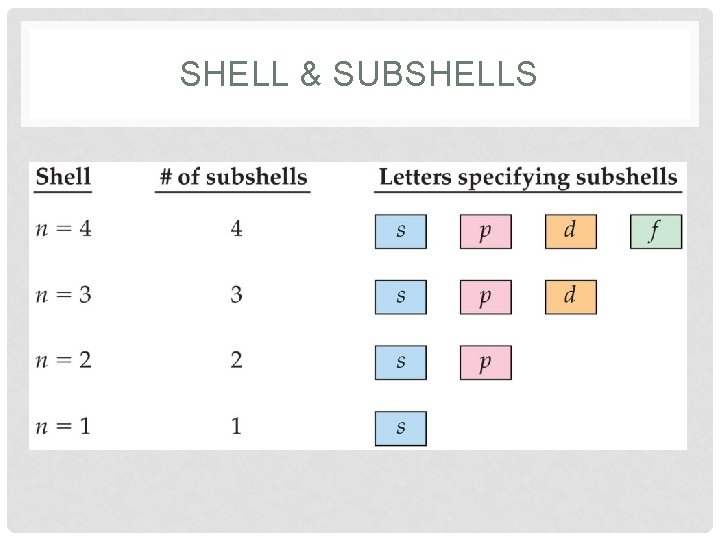
SHELL & SUBSHELLS • Shell • energy level (aka principal quantum number), represented by n • coefficient in an EC • Subshells(Sublevel) energy level • identified by s, p, d, or f in an EC • number of sublevels per shell is equal to the shell number Example 2 p 2 energy sublevel

SHELL & SUBSHELLS

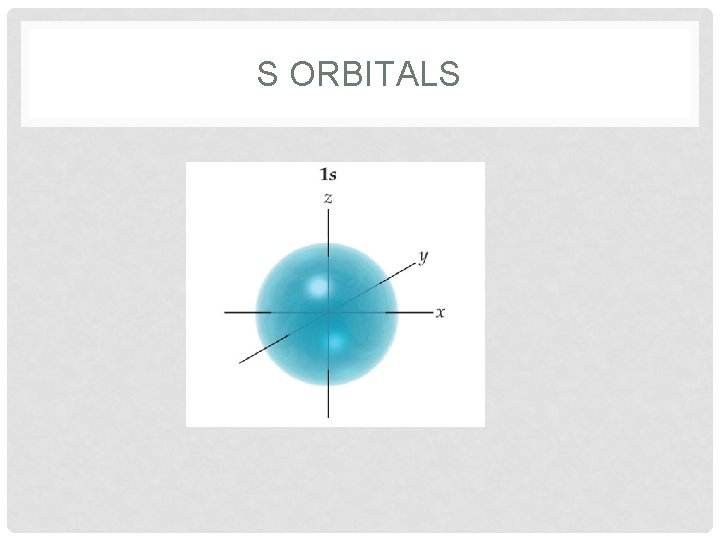
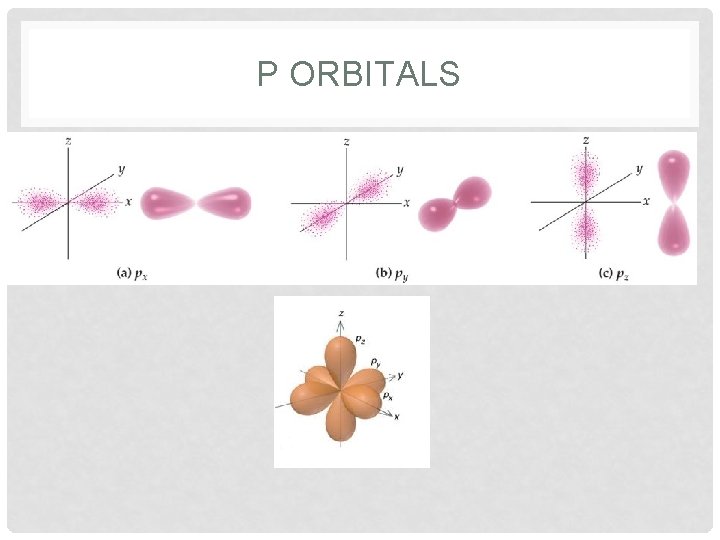
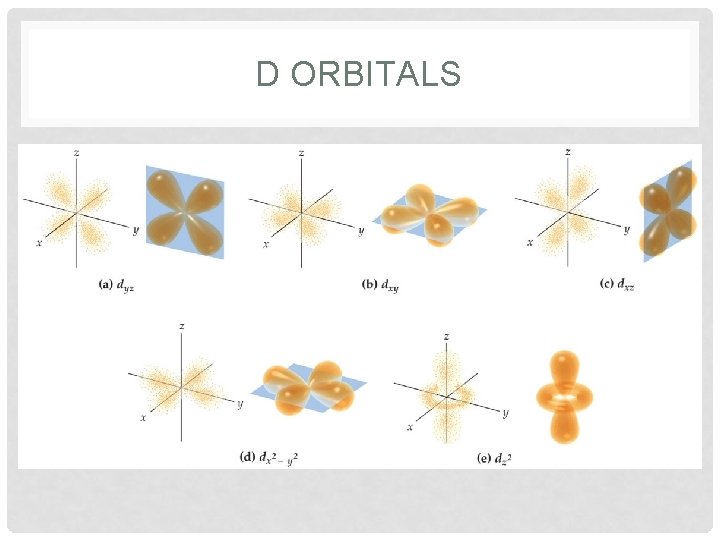
ORBITALS • Orbitals • region where there is the highest probability of finding an electron • each orbital has a different shape • s = sphere • p = peanut • each sublevel also has specific number of orbitals • s = 1 orbital; p = 3 orbitals; d = 5 orbitals; f = 7 orbitals • superscript describes how many electrons are in each orbital

S ORBITALS

P ORBITALS

D ORBITALS

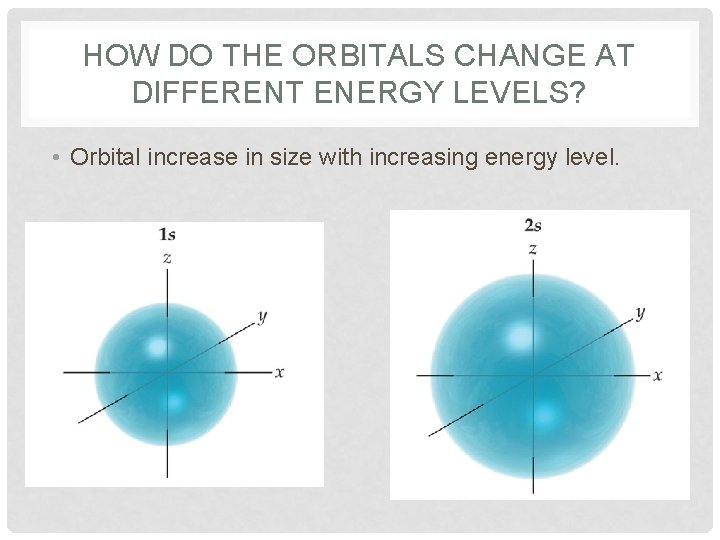
HOW DO THE ORBITALS CHANGE AT DIFFERENT ENERGY LEVELS? • Orbital increase in size with increasing energy level.

BASIC ELECTRON CONFIGURATION PRINCIPLES • Pauli Exclusion Principle • each orbital contains a maximum of two electrons • Aufbau Principle • electrons occupy orbitals of the lowest energy first • Hund’s Rule • every orbital is singly occupied with one electron before any one orbital is doubly occupied

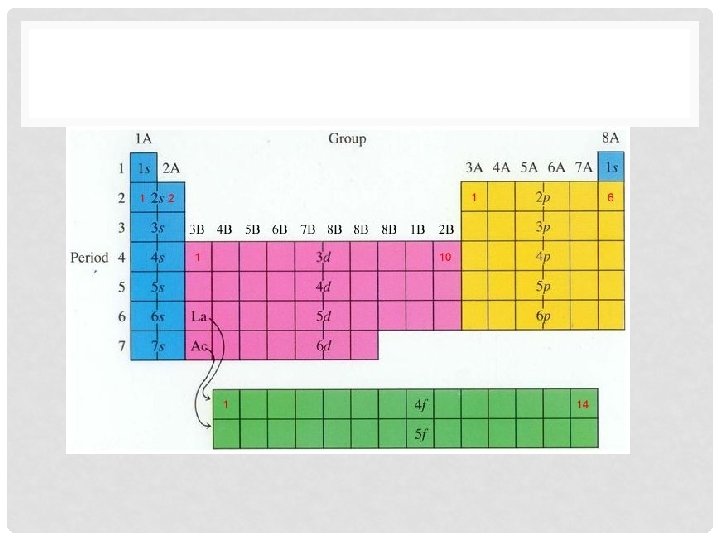
ELECTRON CONFIGURATIONS & THE PERIODIC TABLE • How does the periodic table relate to electron configurations? • Each row represents the shell or energy level. • For example: The first energy level has how many electrons? • n = 1; 1 subshell = s; 1 s-orbital • 2 electrons per orbital gives 2 electrons in the first energy level! • The first row on the periodic table has two elements!!!

CONTINUED • Still not convinced! Let’s look at another example: • The second energy level has how many electrons? • n = 2 ; 2 subshells = s & p; 1 s-orbital & 3 p-orbitals • 2 electrons per orbital gives 8 electrons!!! Whoa!!! That was tough!!!! • The second row on the periodic table has 8 elements!!!!

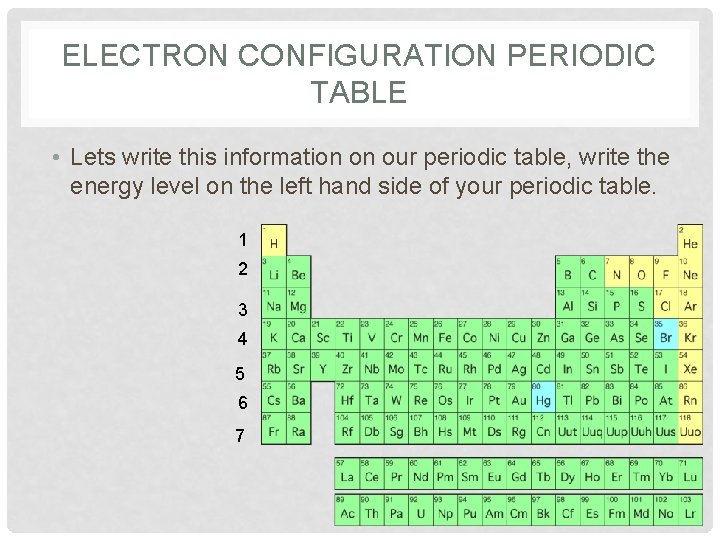
ELECTRON CONFIGURATION PERIODIC TABLE • Lets write this information on our periodic table, write the energy level on the left hand side of your periodic table. 1 2 3 4 5 6 7

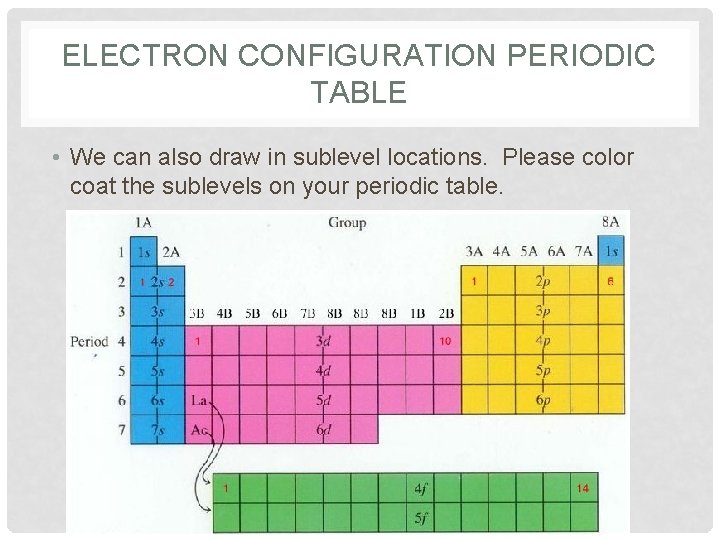
ELECTRON CONFIGURATION PERIODIC TABLE • We can also draw in sublevel locations. Please color coat the sublevels on your periodic table.

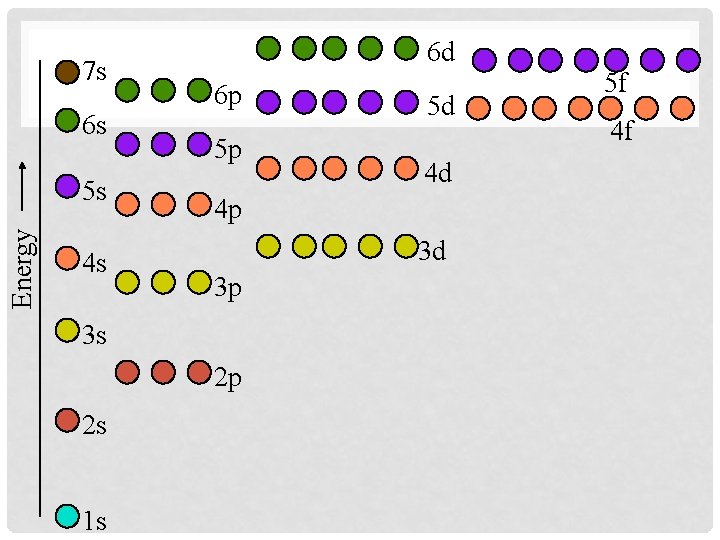
PERIODIC TABLE PROBLEM • If we look at n=3, there are only 8 elements in the row but there are suppose to be 18 electrons in the energy level. (You should be able to figure out the number of electrons!!) • As you increase the energy level the electrons tend to overlap in complicated ways. • The “d” subshell is actually one less than the row it is in.

7 s 6 s Energy 5 s 4 s 6 d 6 p 5 p 3 d 3 p 2 p 1 s 4 d 4 p 3 s 2 s 5 d 5 f 4 f


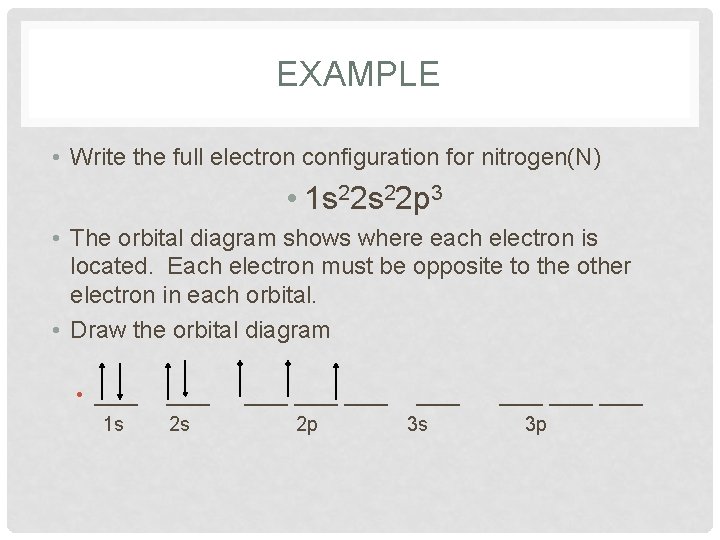
EXAMPLE • Write the full electron configuration for nitrogen(N) • 1 s 22 p 3 • The orbital diagram shows where each electron is located. Each electron must be opposite to the other electron in each orbital. • Draw the orbital diagram • ____ 1 s ____ 2 s ____ 2 p 3 s ____ 3 p

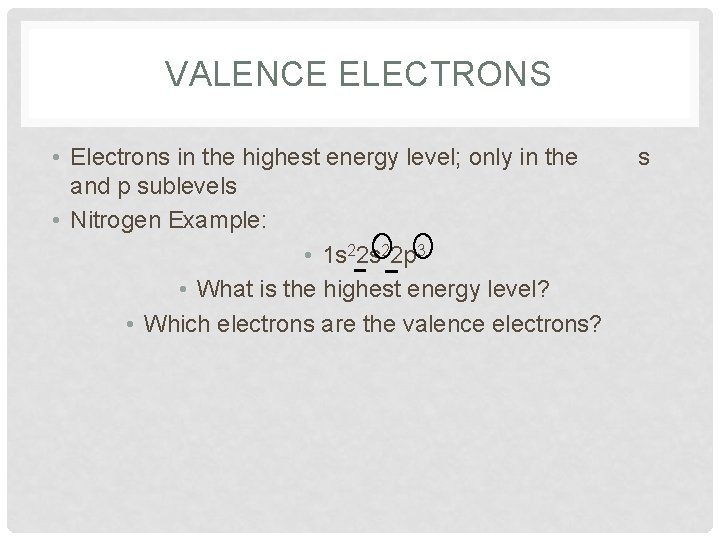
VALENCE ELECTRONS • Electrons in the highest energy level; only in the and p sublevels • Nitrogen Example: • 1 s 22 p 3 • What is the highest energy level? • Which electrons are the valence electrons? s