Chapter 4 Arrangement of Electrons in Atoms 4




















- Slides: 48

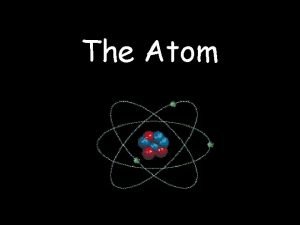
Chapter 4 Arrangement of Electrons in Atoms 4. 1 The Development of a New Atomic Model

Properties of Light • Electromagnetic Radiation: EM radiation are forms of energy which move through space as waves ! e r o m • There are many different types cofh AEM ny waves ea B e h T light r o • visible F t us J t o N : s e v • x-rays Wa • ultraviolet light • infrared light • radio waves

EM Waves • Move at speed of light: 3. 00 x 108 m/s • Speed is equal to the frequency times the wavelength c = v • Frequency (v) is the number of waves passing a given point in one second • Wavelength ( ) is the distance between peaks of adjacent waves • Speed of light is a constant, so v is also a constant; v and must be inversely proportional


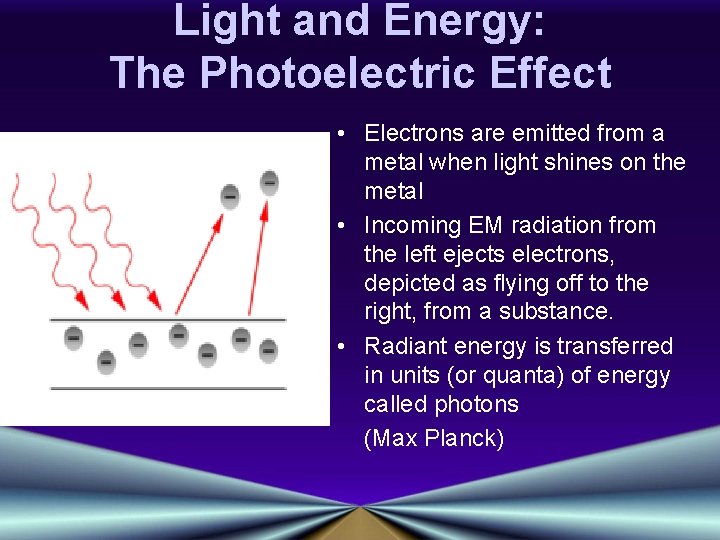
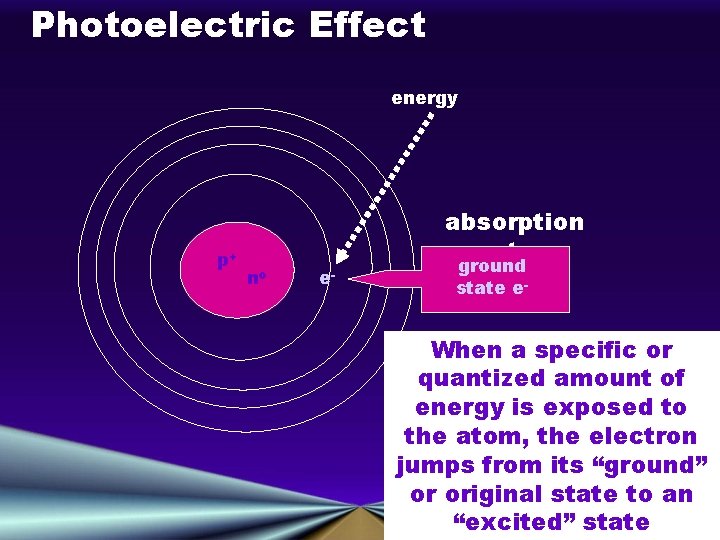
Light and Energy: The Photoelectric Effect • Electrons are emitted from a metal when light shines on the metal • Incoming EM radiation from the left ejects electrons, depicted as flying off to the right, from a substance. • Radiant energy is transferred in units (or quanta) of energy called photons (Max Planck)

Photoelectric Effect energy p+ no e- absorption spectrum ground state e- When a specific or quantized amount of energy is exposed to the atom, the electron jumps from its “ground” or original state to an “excited” state

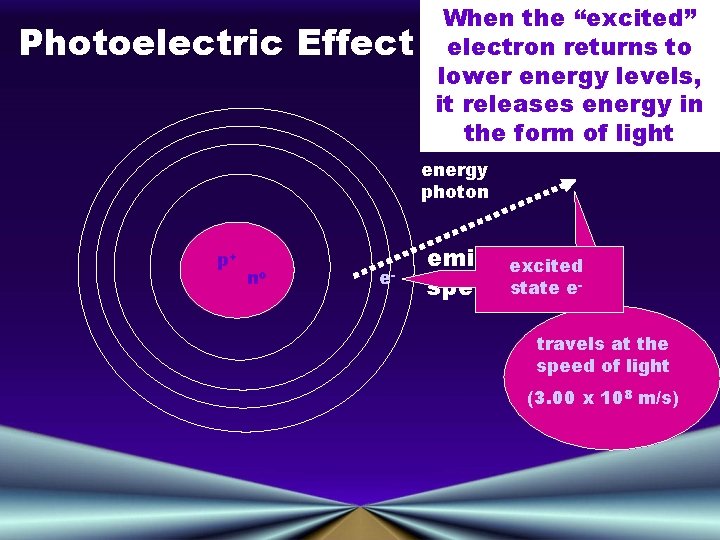
Photoelectric Effect When the “excited” electron returns to lower energy levels, it releases energy in the form of light energy photon p+ no e- emission excited state espectrum! travels at the speed of light (3. 00 x 108 m/s)

• A photon is a particle of energy having a rest mass of zero and carrying a quantum of energy • A quantum is the minimum amount of energy that can be lost or gained by an atom • Energy of a photon is directly proportional to the frequency of radiation • E = hv (h is Planck’s constant, 6. 62554 x 10 -24 J * sec)

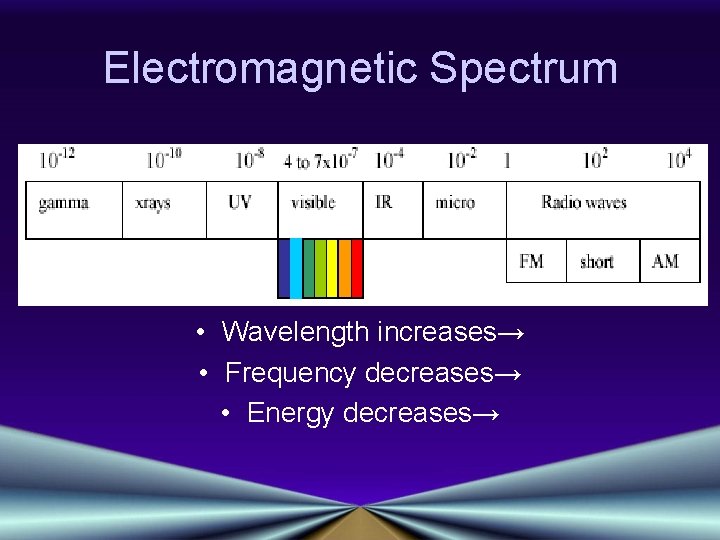
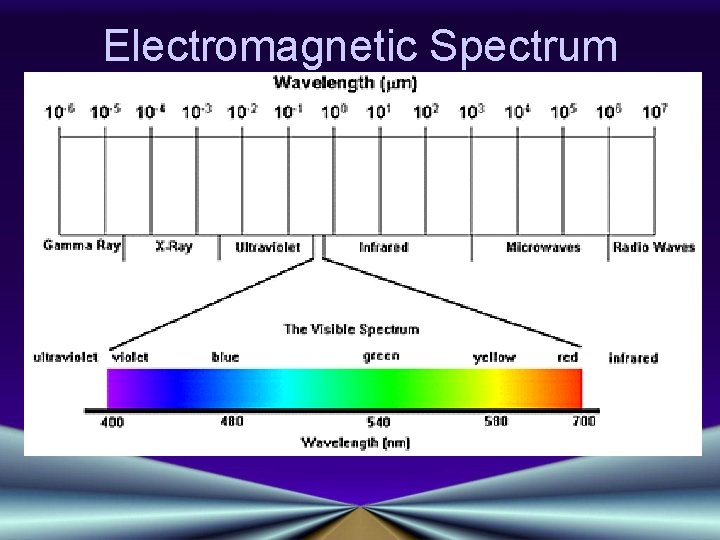
Electromagnetic Spectrum • Wavelength increases→ • Frequency decreases→ • Energy decreases→

Electromagnetic Spectrum

Wave-Particle Duality • Energy travels through space as waves, but can be thought of as a stream of particles (Einstein) • Each particle has 1 quantum of energy.

Line Spectrums • Ground State: The lowest energy state of an atom • Excited State: A state in which an atom has a higher potential energy than in its ground state example: Neon lights

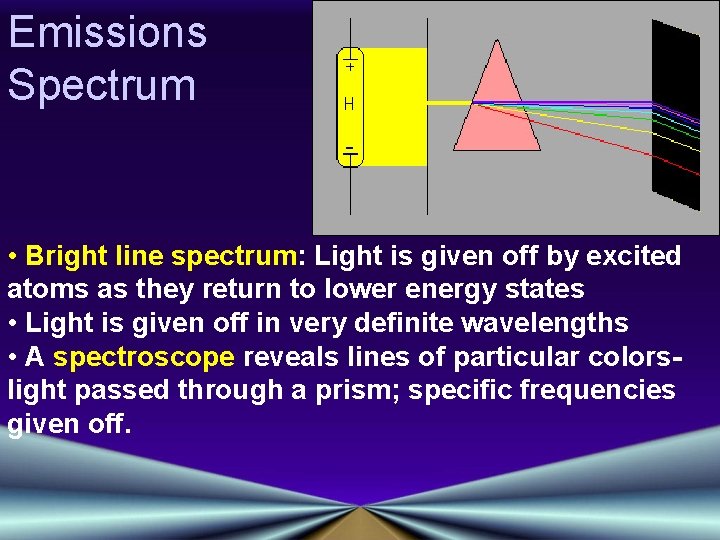
Emissions Spectrum • Bright line spectrum: Light is given off by excited atoms as they return to lower energy states • Light is given off in very definite wavelengths • A spectroscope reveals lines of particular colorslight passed through a prism; specific frequencies given off.

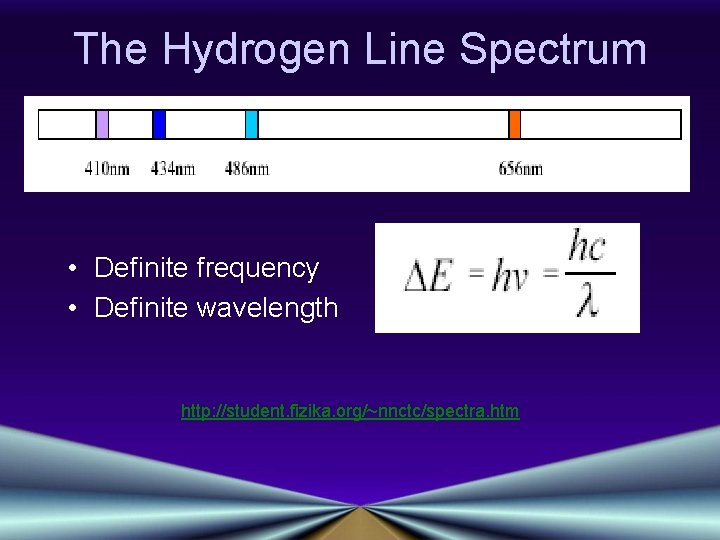
The Hydrogen Line Spectrum • Definite frequency • Definite wavelength http: //student. fizika. org/~nnctc/spectra. htm

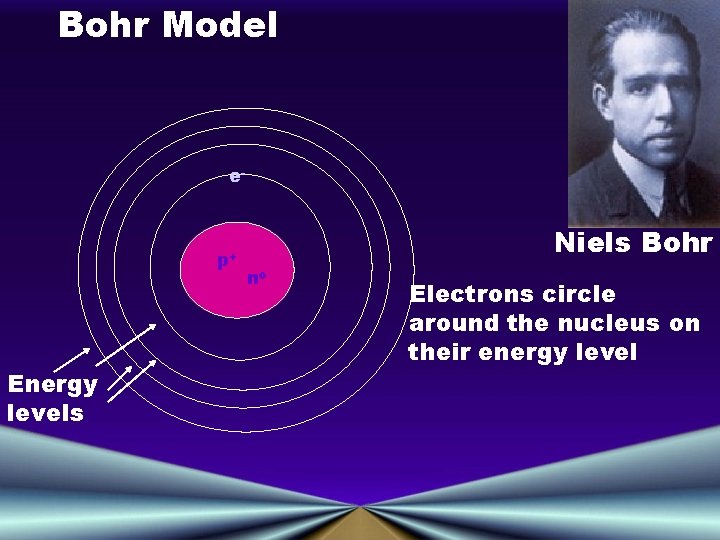
Bohr Model e- p+ Energy levels Niels Bohr no Electrons circle around the nucleus on their energy level

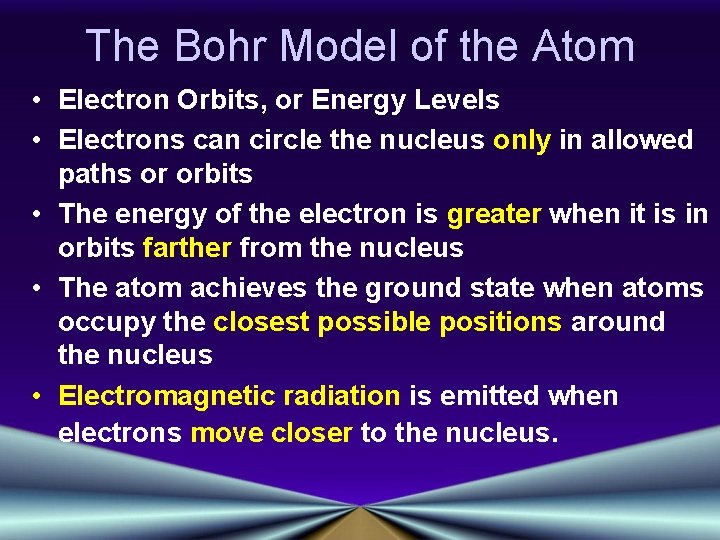
The Bohr Model of the Atom • Electron Orbits, or Energy Levels • Electrons can circle the nucleus only in allowed paths or orbits • The energy of the electron is greater when it is in orbits farther from the nucleus • The atom achieves the ground state when atoms occupy the closest possible positions around the nucleus • Electromagnetic radiation is emitted when electrons move closer to the nucleus.

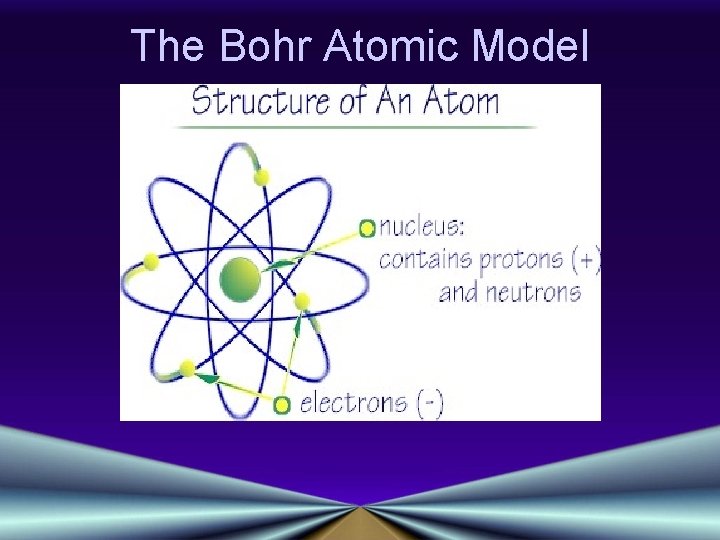
The Bohr Atomic Model

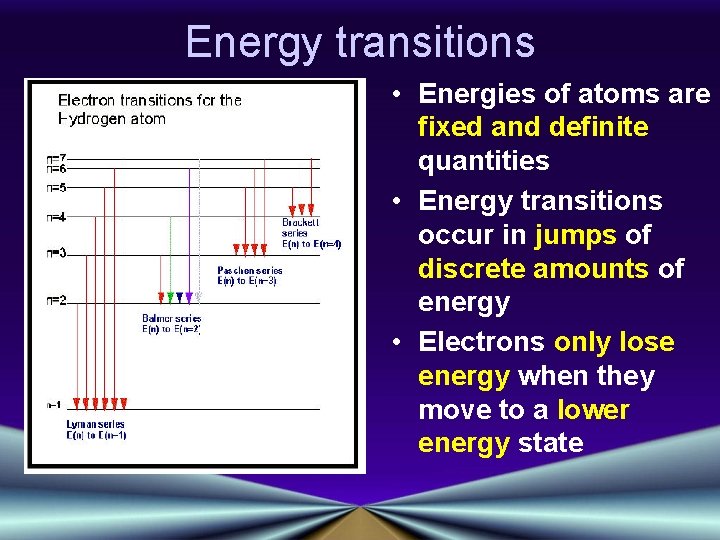
Energy transitions • Energies of atoms are fixed and definite quantities • Energy transitions occur in jumps of discrete amounts of energy • Electrons only lose energy when they move to a lower energy state

Shortcomings of the Bohr Model • Doesn't work for atoms larger than hydrogen (more than one electron) • Doesn't explain chemical behavior

Chapter 4 Arrangement of Electrons in Atoms 4. 2 The Quantum Model of the Atom

Electrons as Waves and Particles • Louis de. Broglie (1924) • Electrons have wavelike properties • Consider the electron as a wave confined to a space that can have only certain frequencies

The Heisenberg Uncertainty Principle "It is impossible to determine simultaneously both the position and velocity of an electron or any other particle. ” Werner Heisenberg- 1927 • Electrons are located by their interactions with photons • Electrons and photons have similar energies • Interaction between a photon and an electron knocks the electron off of its course

The SchrØdinger Wave Equation • Proved quantization of electron energies and is the basis for Quantum Theory • Quantum theory describes mathematically the wave properties of electrons and other very small particles • Electrons do not move around the nucleus in "planetary orbits" ? ? ? ?

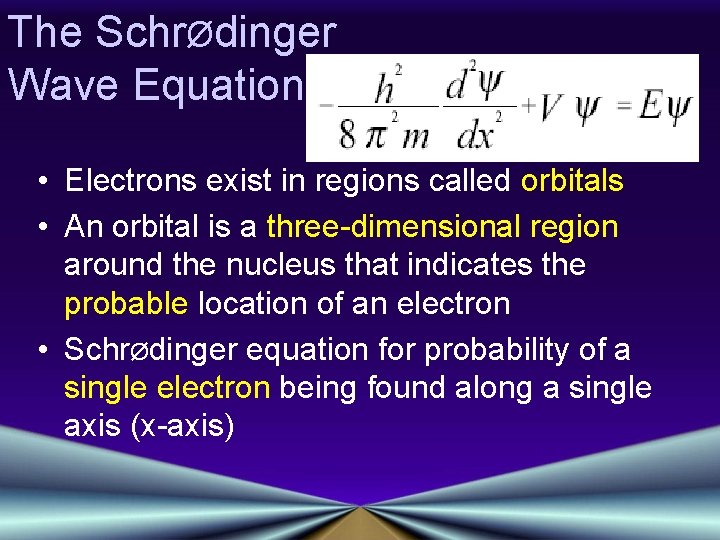
The SchrØdinger Wave Equation • Electrons exist in regions called orbitals • An orbital is a three-dimensional region around the nucleus that indicates the probable location of an electron • SchrØdinger equation for probability of a single electron being found along a single axis (x-axis)

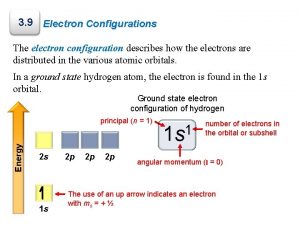
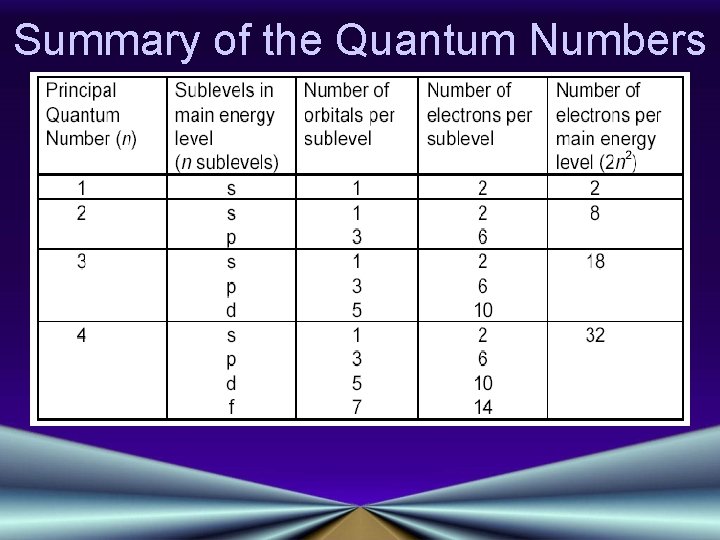
Atomic Orbitals & Quantum Numbers • Quantum Numbers specify the properties of atomic orbitals and the properties of the electrons in orbitals: Principal Quantum Number (n) Angular Momentum Quantum Number (l) Magnetic Quantum Number (m) Spin Quantum Number

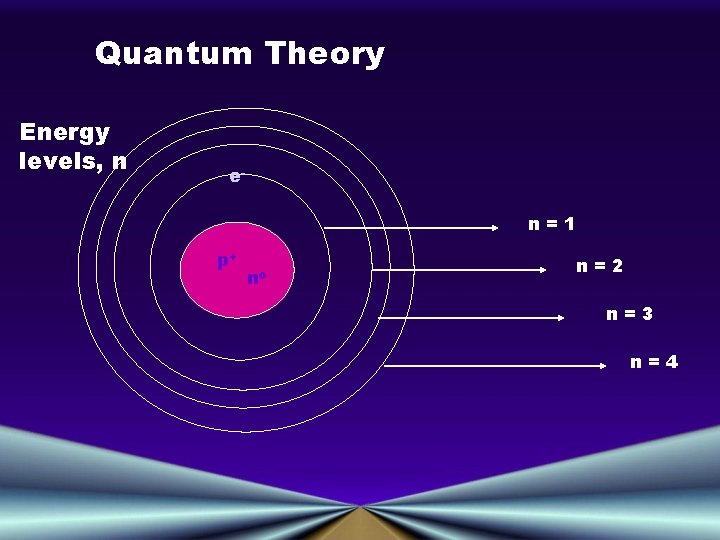
Principal Quantum Number (n) • Indicates the main energy levels occupied by the electron • Values of n are positive integers • n = 1 is closest to the nucleus, and lowest in energy • The number of orbitals possible per energy level (or "shell") is equal to n 2

Quantum Theory Energy levels, n en=1 p+ no n=2 n=3 n=4

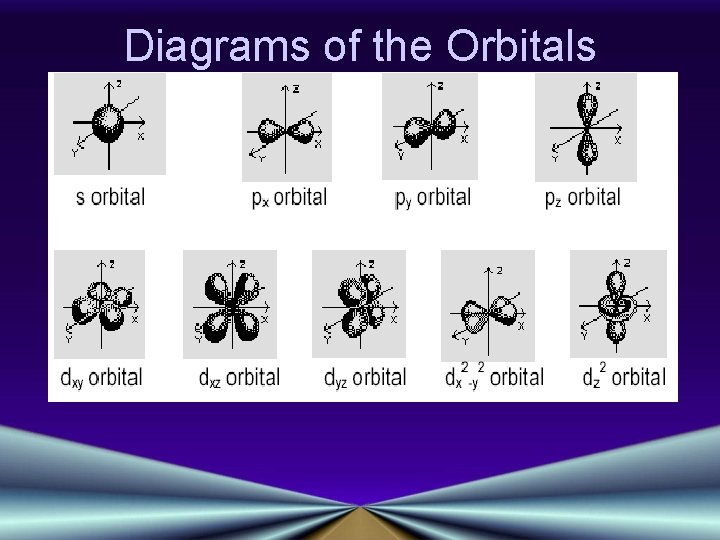
Angular Momentum Quantum Number (l) • • Indicates the shape of the orbital Number of orbital shapes = n Possible values are l = 0, 1, 2, or 3 Shapes are designated s, p, d, f Click Here! • S shape is spherical • P shape is a dumbbell, or figure 8

Magnetic Quantum Number (m) • The orientation of the orbital around the nucleus • s orbitals have only one possible orientation, m=0 • p orbitals have three possible, m = +1, 0 or -1 • d orbitals have five possible, m = -2, -1, 0, +1, or +2 • f orbitals have 7 possible orientations

Spin Quantum Number • Indicates the fundamental spin states of an electron in an orbital • Two possible values for spin, +1/2, -1/2 • A single orbital can contain only two electrons, which must have opposite spins

Diagrams of the Orbitals

Summary of the Quantum Numbers

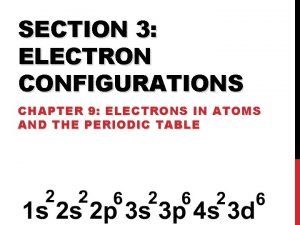
Chapter 4 Arrangement of Electrons in Atoms 4. 3 Electron Configurations

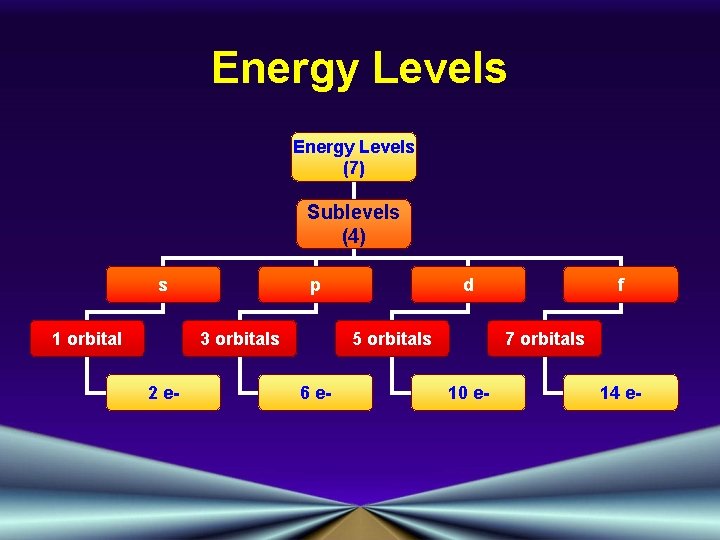
Energy Levels (7) Sublevels (4) s 1 orbital p 3 orbitals 2 e- d 5 orbitals 6 e- f 7 orbitals 10 e- 14 e-

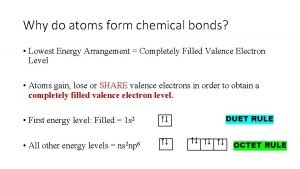
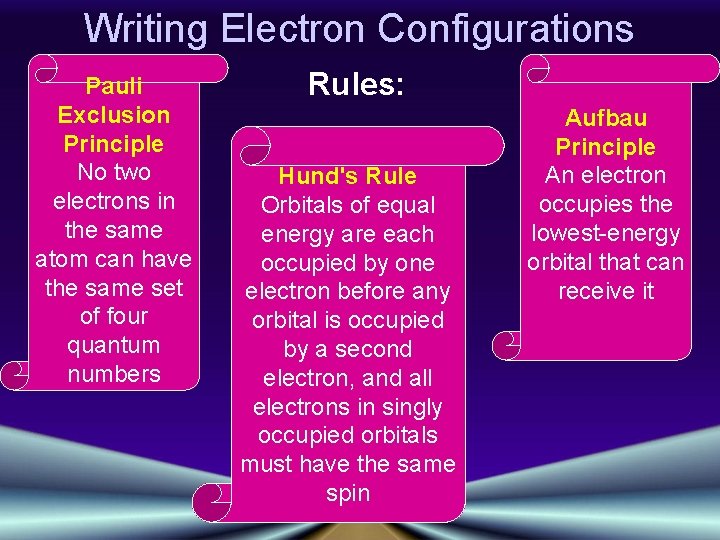
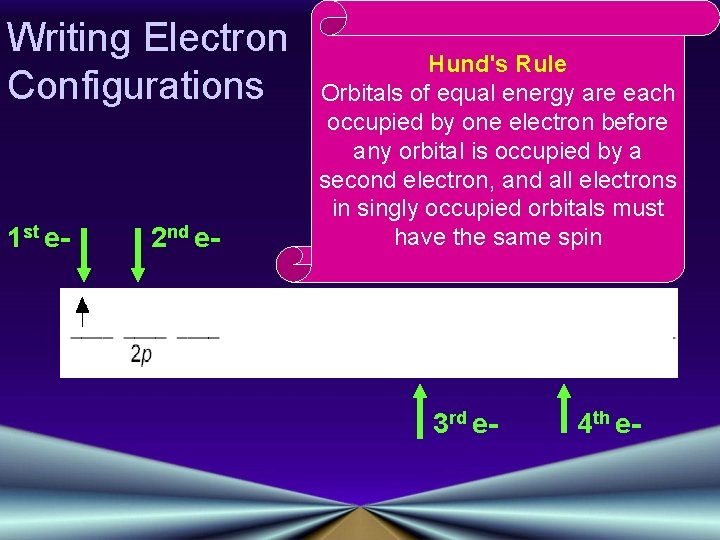
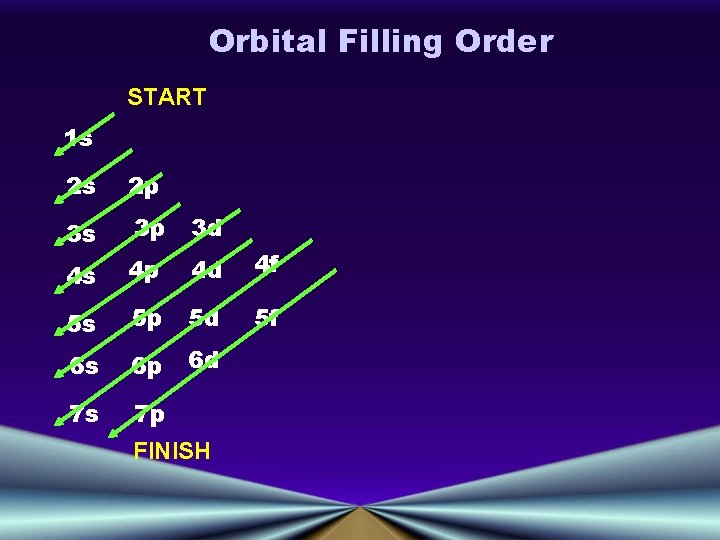
Writing Electron Configurations Pauli Exclusion Principle No two electrons in the same atom can have the same set of four quantum numbers Rules: Hund's Rule Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin Aufbau Principle An electron occupies the lowest-energy orbital that can receive it

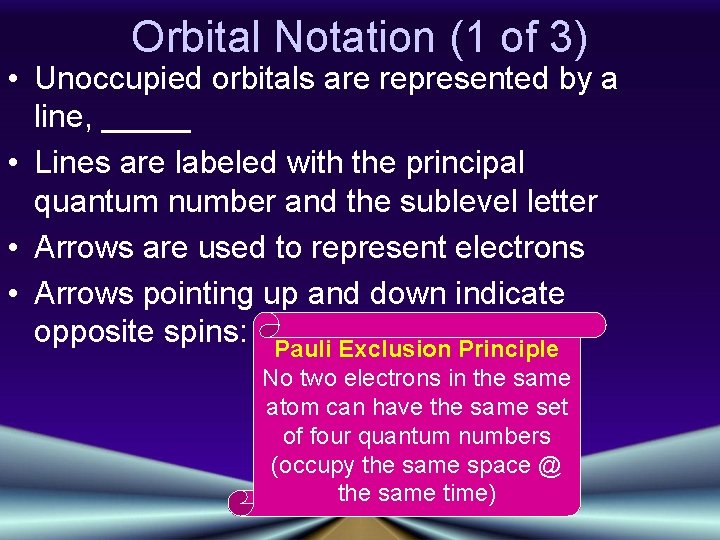
Orbital Notation (1 of 3) • Unoccupied orbitals are represented by a line, _____ • Lines are labeled with the principal quantum number and the sublevel letter • Arrows are used to represent electrons • Arrows pointing up and down indicate opposite spins: Pauli Exclusion Principle No two electrons in the same atom can have the same set of four quantum numbers (occupy the same space @ the same time)

Writing Electron Configurations 1 st e- 2 nd e- Hund's Rule Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin 3 rd e- 4 th e-

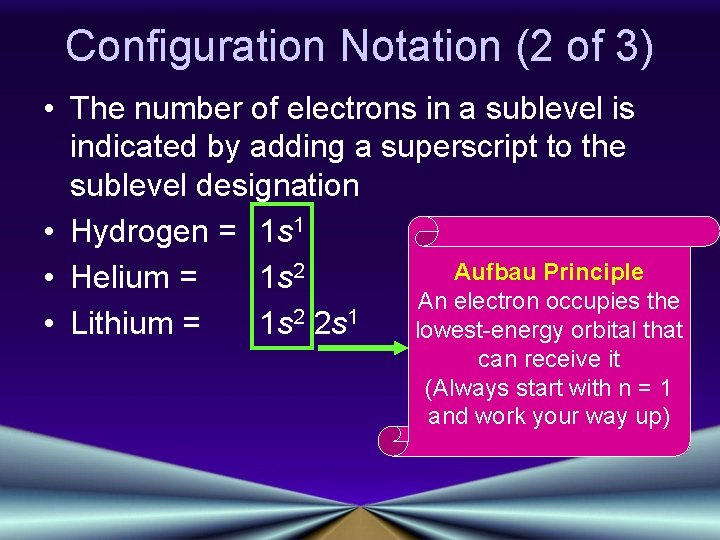
Configuration Notation (2 of 3) • The number of electrons in a sublevel is indicated by adding a superscript to the sublevel designation • Hydrogen = 1 s 1 Aufbau Principle • Helium = 1 s 2 An electron occupies the • Lithium = 1 s 2 2 s 1 lowest-energy orbital that can receive it (Always start with n = 1 and work your way up)

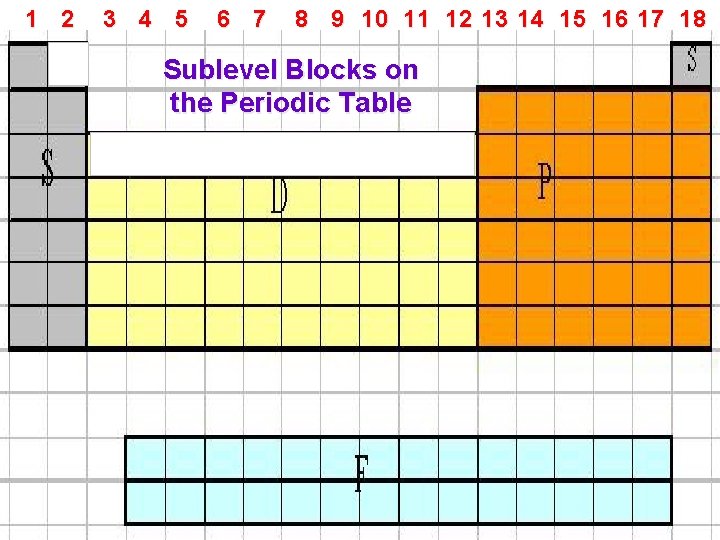
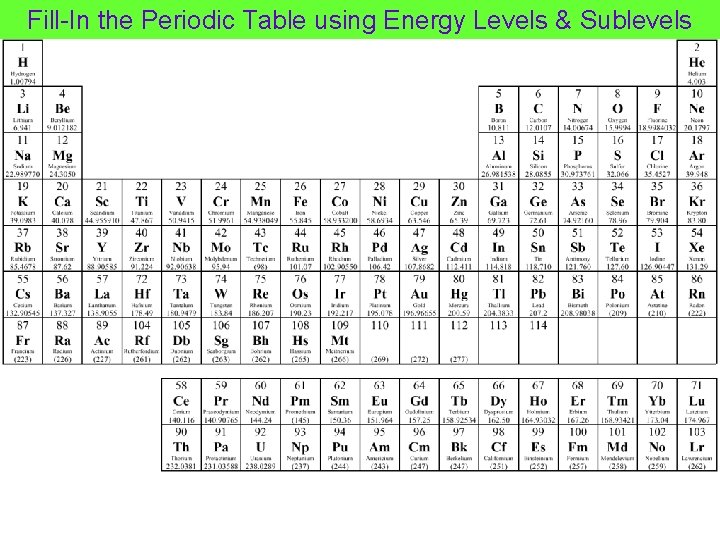
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Sublevel Blocks on the Periodic Table


Fill-In the Periodic Table using Energy Levels & Sublevels

Orbital Filling Order START 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 5 s 5 p 5 d 5 f 6 s 6 p 6 d 7 s 7 p FINISH

Noble Gas Notation (3 of 3) • The configuration begins with the preceding noble gas’s symbol in brackets and is followed by the rest of the configuration for the particular element. • [Ne] 3 s 23 p 5

Terms • Highest occupied energy level: The electron containing energy level with the highest principal quantum number • Inner shell electrons: Electrons that are not in the highest energy level • Octet Rule: Highest energy level s and p electrons are filled (8 electrons)

Octet Rule • Characteristic of noble gases, Group 18 • Exceptions: Hydrogen & Helium • Noble gas configuration: Outer main energy level fully occupied, usually (except for He) by eight electrons This configuration has extra stability

Survey of the Periodic Table Elements of the Fourth Period • Irregularity of Chromium • Expected: • Actual: 1 s 22 p 63 s 23 p 64 s 23 d 4 1 s 22 p 63 s 23 p 64 s 13 d 5 Rule: Sublevels are most stable when they are either half or completely filled. Electrons will shift to different energy levels to accommodate this stability whenever possible.

Survey of the Periodic Table • Several transition and rare-earth elements borrow from smaller sublevels in order to half fill larger sublevels i. e. d borrows 1 e- from s • This accounts for some of the unexpected electron configurations found within the transition elements.

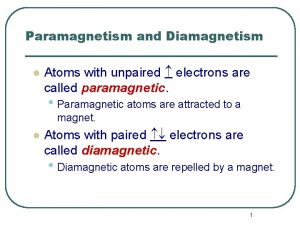
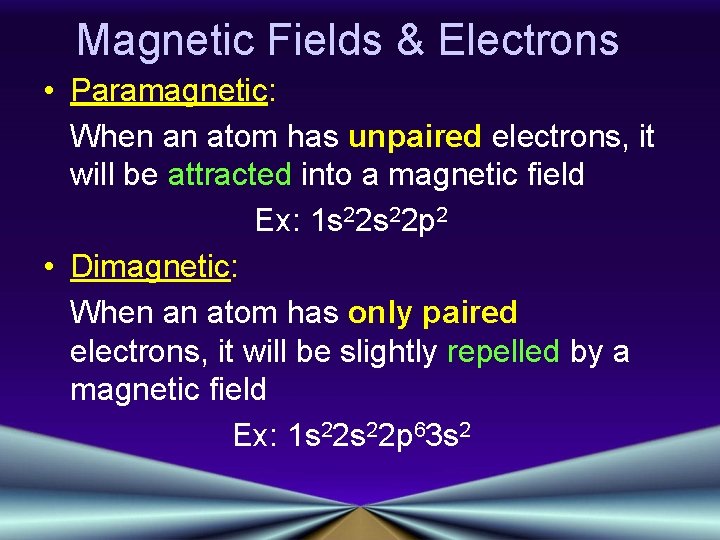
Magnetic Fields & Electrons • Paramagnetic: When an atom has unpaired electrons, it will be attracted into a magnetic field Ex: 1 s 22 p 2 • Dimagnetic: When an atom has only paired electrons, it will be slightly repelled by a magnetic field Ex: 1 s 22 p 63 s 2
 Arrangement of electrons in atoms chapter 4 test
Arrangement of electrons in atoms chapter 4 test Chapter 5 review arrangement of electrons in atoms
Chapter 5 review arrangement of electrons in atoms Ccechs
Ccechs Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Regents periodic table
Regents periodic table Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Atoms with 4 valence electrons
Atoms with 4 valence electrons Atoms with unpaired electrons are called diamagnetic.
Atoms with unpaired electrons are called diamagnetic. How to find the electrons in periodic table
How to find the electrons in periodic table Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom What is the oxidation number of lithium
What is the oxidation number of lithium How do chemists model the valence electrons of metal atoms?
How do chemists model the valence electrons of metal atoms? How do chemists model the valence electrons of metal atoms?
How do chemists model the valence electrons of metal atoms? Copper subshell configuration
Copper subshell configuration 5 electrons in atoms
5 electrons in atoms Unstable arrangement of atoms
Unstable arrangement of atoms Arrangement of electrons
Arrangement of electrons Hund's rule vs pauli exclusion principle
Hund's rule vs pauli exclusion principle Copper subshell configuration
Copper subshell configuration Chapter 4 section 2 the structure of atoms
Chapter 4 section 2 the structure of atoms Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Chapter 6 electronic structure of atoms answers
Chapter 6 electronic structure of atoms answers Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Which of the d orbitals most resembles a pz orbital?
Which of the d orbitals most resembles a pz orbital? Chapter 3 atoms the building blocks of matter
Chapter 3 atoms the building blocks of matter Which subatomic particle has the least mass
Which subatomic particle has the least mass Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Arrangement of organisms
Arrangement of organisms Why do atoms react
Why do atoms react Which atoms have expanded octet
Which atoms have expanded octet What is the relationship between atoms and elements
What is the relationship between atoms and elements What are atoms?
What are atoms? Kesler science biotic and abiotic factors answer key
Kesler science biotic and abiotic factors answer key Matterville answer key
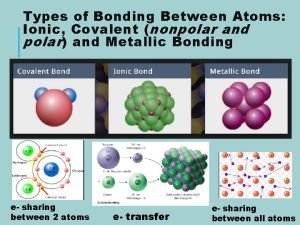
Matterville answer key Ionic metallic and covalent bonds
Ionic metallic and covalent bonds Gfm formula
Gfm formula Atoms in increasing size
Atoms in increasing size The smallest building block of matter
The smallest building block of matter The atoms family song
The atoms family song Whats the nucleus
Whats the nucleus Which atoms has the largest atomic radius
Which atoms has the largest atomic radius How to do grams to moles
How to do grams to moles Atomic mass unit
Atomic mass unit Metallic bonding occurs between atoms of copper
Metallic bonding occurs between atoms of copper Atoms escape room digital locks answers
Atoms escape room digital locks answers Mri hydrogen atoms
Mri hydrogen atoms