Chapter 4 Arrangement of Electrons in Atoms I








- Slides: 13

Chapter 4 Arrangement of Electrons in Atoms

I. The Development of a new Atomic Model A. Properties of light 1. before 1900 light thought to be a wave only 2. Wave description of light a. electromagnetic radiation – b. electromagnetic spectrum – c. wavelength ( – )ג d. frequency (v) – e. c = ג v f. c = 3. 0 x 108 m/s

B. The Photoelectric Effect – 1. Particle description of light a. Max Planck – an object emits energy in small, specific packets of energy b. quantum – c. E = hv d. h = 6. 626 x 10 -34 J*s

e. 1905 Einstein introduced the idea that Electromagnetic radiation has dual waveparticle nature f. E photon = hv C. The Hydrogen Atom Line Emission Spectrum 1. ground state – 2. excited state – 3. line-emission spectrum -

4. continuous spectrum – D. Bohr Model of the Hydrogen Atom 1. 1913 – Bohr proposed that electrons travel in distinct paths 2. now we know these are more like cloud regions see page 103

II. The Quantum Model of the Atom A. Electrons as waves 1. studies of the photoelectric effect showed that light could behave as waves and particles 2. De. Broglie hypothesized: a. electron waves could exist only at specific frequencies b. electrons can be bent or defracted

B. The Heisenberg Uncertainty Principle 1. if electrons are wave-particles then where are they in the atom? ? 2. 1927, German, physicist, Werner Heisenberg determined that electrons are detected by their interaction with photons 3. Heisenberg uncertainty principle -

C. The Schrodinger Wave Equation 1. only waves of specific energies provide solutions to the equation 2. Heisenberg and Schrodinger’s equations laid the foundation for the modern quantum theory 3. Quantum Theory – 4. Orbital -

D. Atomic Orbitals and Quantum Numbers 1. quantum numbers – 2. the first 3 quantum numbers indicate the main E level, the shape, and the orientation of an orbital 3. principal quantum number – 4. angular momentum quantum number – a. s – spherical b. p – dumb-bell c. d – more complex

5. Magnetic quantum number – 6. spin quantum number -

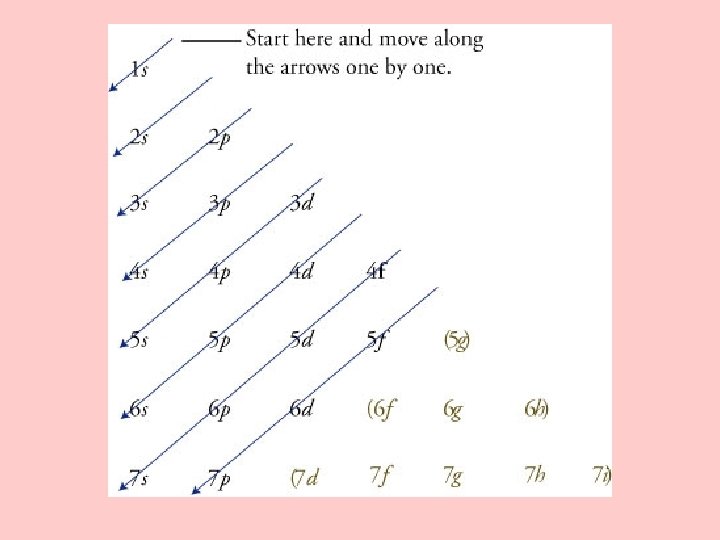
III. Electron Configurations A. electron configuration – B. Rules Governing Electron Configurations 1. Aufbau principle – 2. Pauli Exclusion principle – 3. Hund’s rule -


C. Representing Electron Configurations 1. orbital notation – use arrows 2. electron-configuration notation – use superscripts 3. Noble-Gas Notation-
 Chapter 4 arrangement of electrons in atoms test
Chapter 4 arrangement of electrons in atoms test Chapter 5 review arrangement of electrons in atoms
Chapter 5 review arrangement of electrons in atoms Ccechs
Ccechs Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Periodic table regents
Periodic table regents Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Atoms with 4 valence electrons
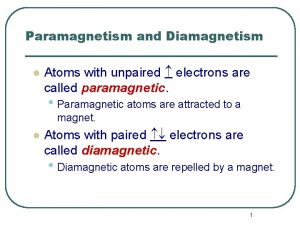
Atoms with 4 valence electrons Atoms with unpaired electrons are called diamagnetic.
Atoms with unpaired electrons are called diamagnetic. How to find protons and electrons
How to find protons and electrons Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy The lowest allowable energy state of an atom is called
The lowest allowable energy state of an atom is called Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Atoms tend to gain lose or share electrons
Atoms tend to gain lose or share electrons