Chapter 4 Arrangement of Electrons in Atoms I


















- Slides: 30

Chapter 4 Arrangement of Electrons in Atoms

I. The Development of a New Atomic Model H Electromagnetic Radiation: H “radiant energy” form of nrg that has wave characteristics and can travel through a vacuum “light” H Electromagnetic Spectrum: H Distribution among various wavelengths of the radiant nrg emitted or absorbed by an object

H Wavelength ( ): corresponding points on adjacent waves---Ex: Frequency ( ): # of waves that pass a point in a specific time H c = ( ) ------inversely proportional

H c = ( ) ------inversely proportional c : m/s : m, cm, nm : waves/second--Hertz (Hz)

H Photoelectric Effect: emission of e- by certain metals when light shines on them

H Quantum: min quantity of nrg that can be lost or gained by an atom H E = (h) ( ) o J = (J s) (Hz) o Planck’s constant: 6. 626 X 10 -34 J s

Video #15 (wave function and wave particle) • Einstein o H dual wave-particle to describe light Photon: radiation with zero mass carrying a quantum of nrg o packet of nrg emitted when an e- drops nrg levels

H Ground state: lowest nrg state H Excited state: higher potential nrg

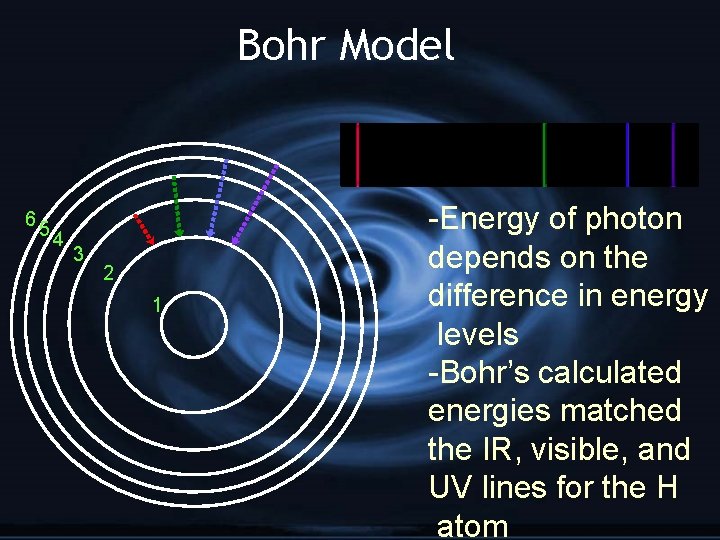
--Bohr’s Model-G e- exist only in orbits with specific amounts of energy called energy levels G Therefore… G e- can only gain or lose certain amounts of energy G only certain photons are produced

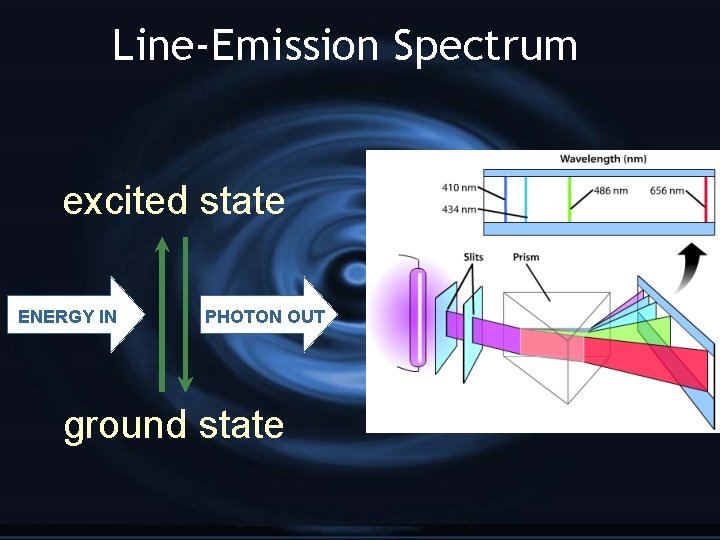
Line-Emission Spectrum excited state ENERGY IN PHOTON OUT ground state

Bohr Model 65 4 3 2 1 -Energy of photon depends on the difference in energy levels -Bohr’s calculated energies matched the IR, visible, and UV lines for the H atom

Other Elements G Each element has a unique bright-line emission spectrum. G “Atomic Fingerprint” Helium z. Bohr’s calculations only worked for hydrogen! ----pg 97

II. The Quantum Model of the Atom G A. Electrons as Waves o Diffraction: bending of a wave as it passes by the edge of an object o Interference: results when waves overlap

EVIDENCE: DIFFRACTION PATTERNS VISIBLE LIGHT ELECTRONS

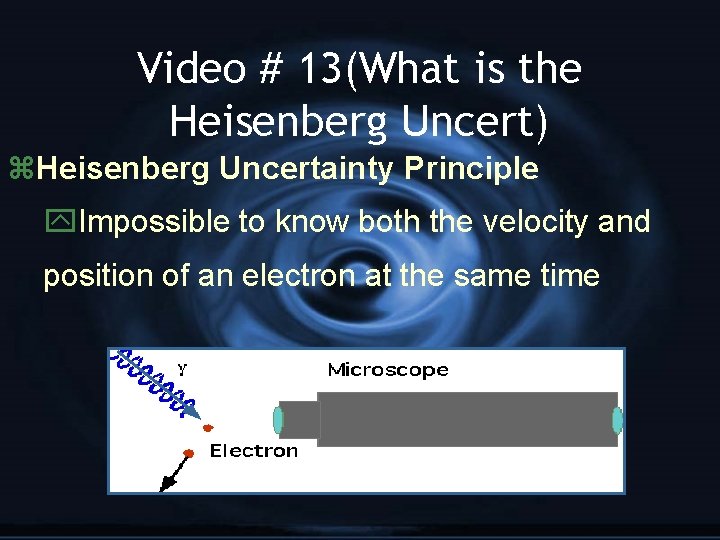
Video # 13(What is the Heisenberg Uncert) z. Heisenberg Uncertainty Principle y. Impossible to know both the velocity and position of an electron at the same time

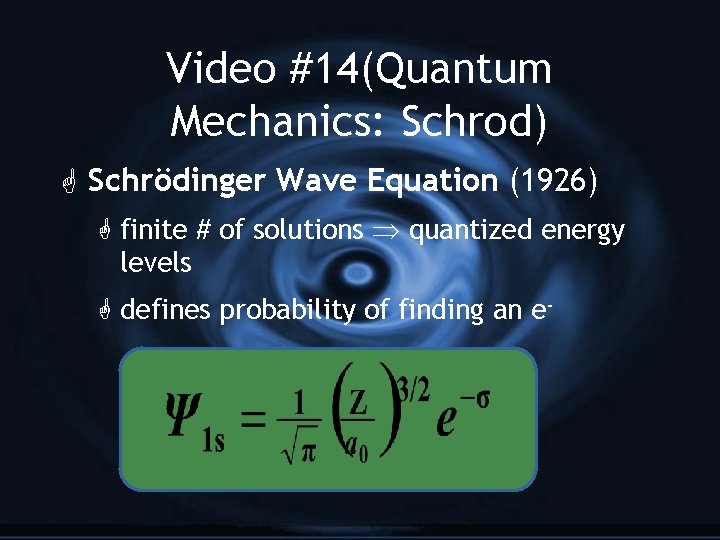
Video #14(Quantum Mechanics: Schrod) G Schrödinger Wave Equation (1926) G finite # of solutions quantized energy levels G defines probability of finding an e-

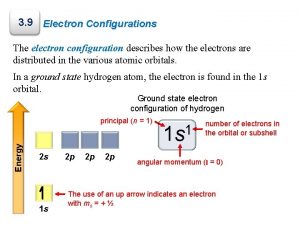
A. Atomic Orbitals and Quantum Numbers G Orbital: probable location of an e. G Quantum #: properties of atomic orbitals and properties of e-’s in orbitals G Principal quantum #: (n), indicates main nrg level occupied by the eo n = 1 -----occupies 1 st nrg level

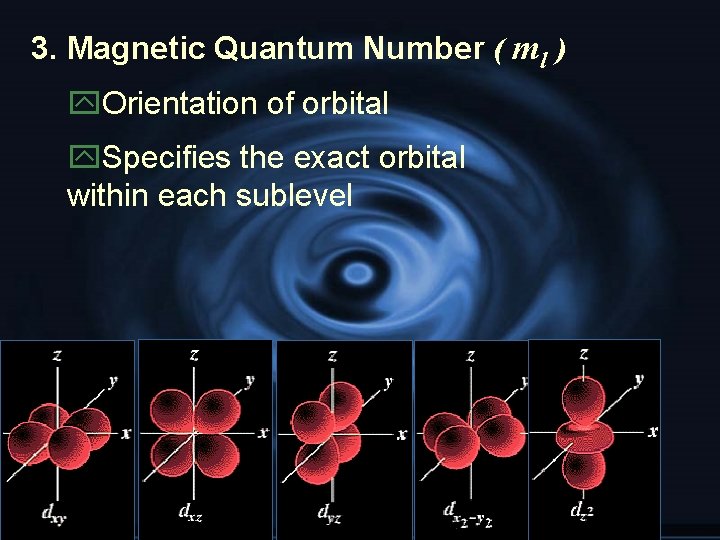
G Angular momentum quantum #: (l), indicates shape of orbital G Magnetic quantum #: (m), orientation of an orbital G Spin quantum #: which spin state (+)(-) G ***See table 4 -2 pg 104

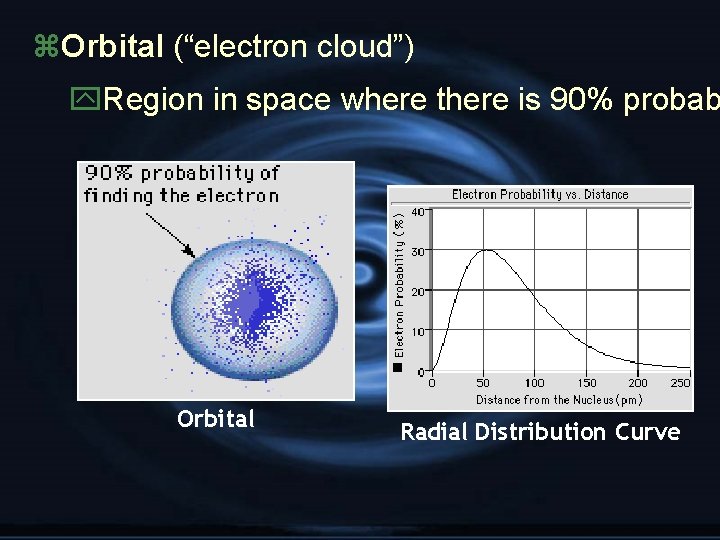
z. Orbital (“electron cloud”) y. Region in space where there is 90% probab Orbital Radial Distribution Curve

z. Four Quantum Numbers: y. Specify the “address” of each electron in an UPPER LEVEL

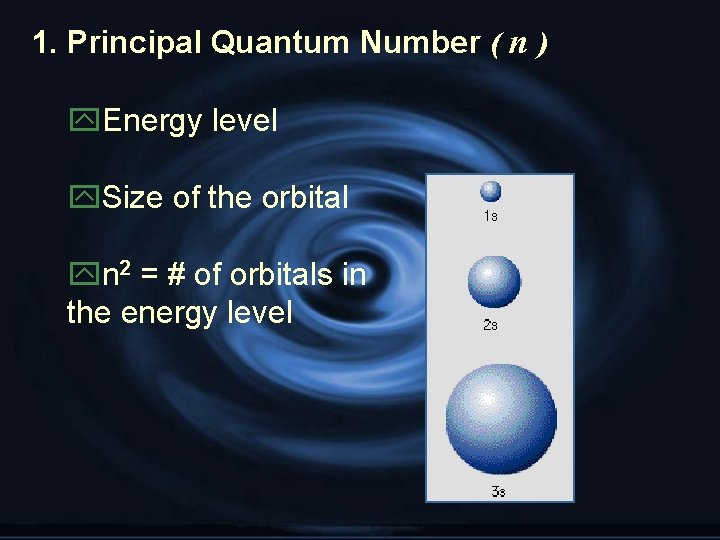
1. Principal Quantum Number ( n ) y. Energy level y. Size of the orbital yn 2 = # of orbitals in the energy level

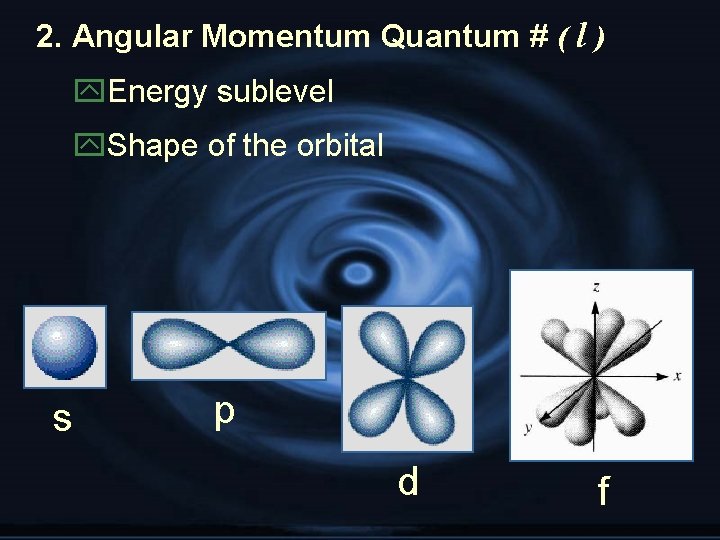
2. Angular Momentum Quantum # ( l ) y. Energy sublevel y. Shape of the orbital s p d f

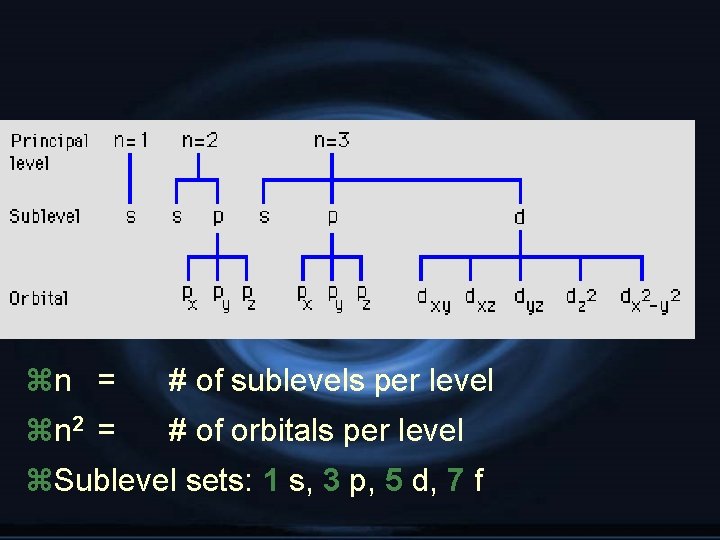
zn = # of sublevels per level zn 2 = # of orbitals per level z. Sublevel sets: 1 s, 3 p, 5 d, 7 f

3. Magnetic Quantum Number ( ml ) y. Orientation of orbital y. Specifies the exact orbital within each sublevel

4. Spin Quantum Number ( ms ) y. Electron spin +½ or -½ y. An orbital can hold 2 electrons that spin in opposite directions.

III. Electron Configuration G Aufbau principle: lowest nrg orbits fill first G Pauli exclusion principle: no 2 e-’s can have the same 4 quantum #’s. This is where spin allows 2 e-’s to be in the same orbit o Ex:

G Hund’s rule: orbital of equal nrg are occupied by 1 e-, before any is occupied by 2 e-’s o Ex: G Orbital Notation: ex: pg 107 G Electron Config Notation: pg 107 G Electron Dot diagram: ex

G Noble gases: G are inert G complete octet G --show ex----

G Table 4 -3 pg 110 1. 2. 3. 4. Principal # energy level Ang. Mom. # sublevel (s, p, d, f) Magnetic # orbital Spin # electron

Feeling overwhelmed?
 Chapter 4 arrangement of electrons in atoms
Chapter 4 arrangement of electrons in atoms Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Ccechs
Ccechs Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Periodic table regents
Periodic table regents Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Atoms with 4 valence electrons
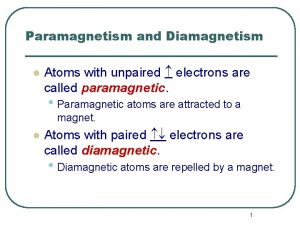
Atoms with 4 valence electrons Diamagnetic elements
Diamagnetic elements How to find protons
How to find protons Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Aluminum chloride charge
Aluminum chloride charge How do chemists model the valence electrons of metal atoms?
How do chemists model the valence electrons of metal atoms? How do chemists model the valence electrons of metal atoms?
How do chemists model the valence electrons of metal atoms? S orbital
S orbital 5 electrons in atoms
5 electrons in atoms Unstable arrangement of atoms
Unstable arrangement of atoms Electronic configuration is arrangement of electrons in
Electronic configuration is arrangement of electrons in Electronic configuration
Electronic configuration Orbital diagram for ca
Orbital diagram for ca Chapter 4 section 2 the structure of atoms
Chapter 4 section 2 the structure of atoms Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Chapter 6 electronic structure of atoms answers
Chapter 6 electronic structure of atoms answers Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Electronic structure of atoms
Electronic structure of atoms Chapter 3 atoms the building blocks of matter
Chapter 3 atoms the building blocks of matter Which subatomic particle has the least mass
Which subatomic particle has the least mass Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Arrangement of organisms
Arrangement of organisms