Chapter 4 Arrangement of Electrons in Atoms Light








- Slides: 33

Chapter 4 Arrangement of Electrons in Atoms

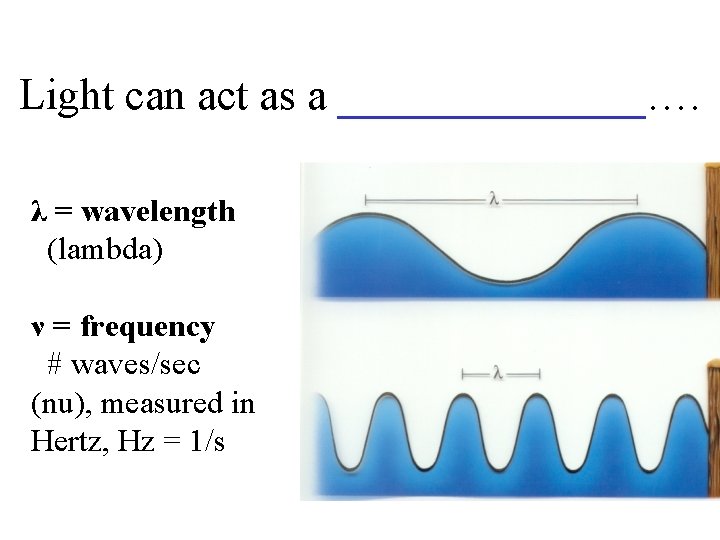
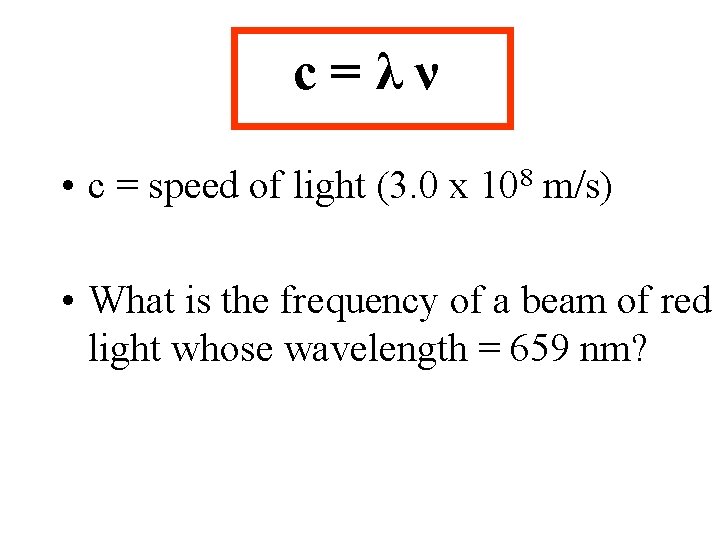
Light can act as a _______…. λ = wavelength (lambda) ν = frequency # waves/sec (nu), measured in Hertz, Hz = 1/s

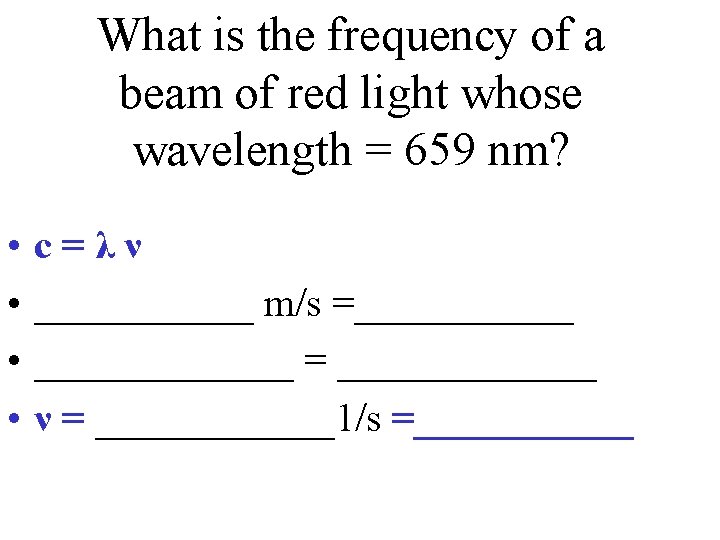
c=λν • c = speed of light (3. 0 x 108 m/s) • What is the frequency of a beam of red light whose wavelength = 659 nm?

What is the frequency of a beam of red light whose wavelength = 659 nm? • • c=λν ______ m/s =_______ = _______ ν = ______1/s =______

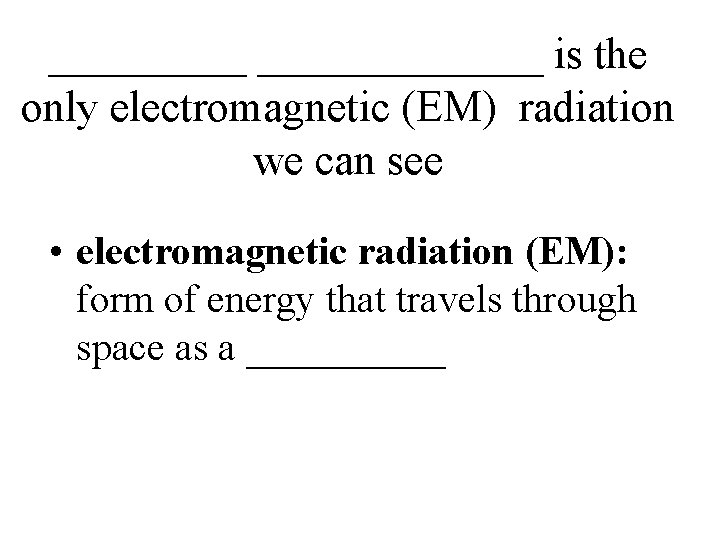
_______ is the only electromagnetic (EM) radiation we can see • electromagnetic radiation (EM): form of energy that travels through space as a _____

Light can also act as a ______ • Supported by 2 experiments: – Photoelectric effect – Hydrogen atom spectrum

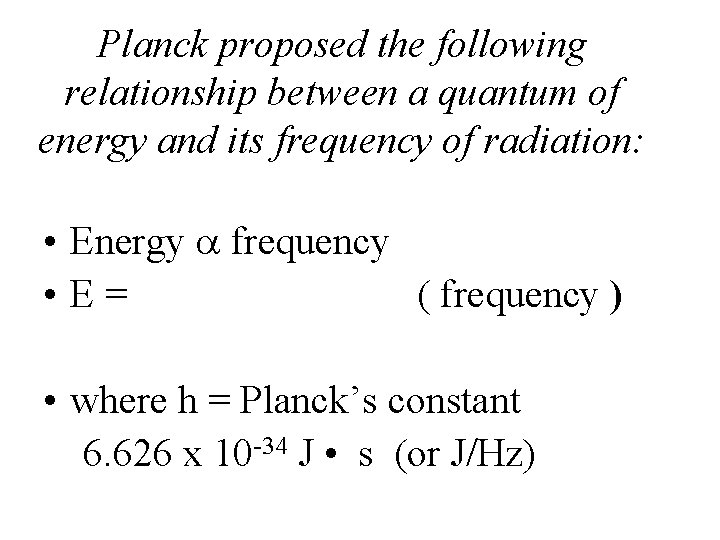
Particle description of light • Max Planck, 1900 – studied ______ of light by hot objects • Proposed that energy is not ______ continuously but in small little ______ called _______ – This is a particle property • “quantum”: min am’t of energy than can be lost or gained by an atom • Energy ________

Planck proposed the following relationship between a quantum of energy and its frequency of radiation: • Energy frequency • E= ( frequency ) • where h = Planck’s constant 6. 626 x 10 -34 J • s (or J/Hz)

Duality of light explained photoelectric effect! Metal must be struck by a photon possessing a min. am’t of E – below this am’t, the electron won’t leave the metal! More light of the same E (same ν) just released more electrons…not more energetic

• Ground state: atoms whose electrons are in their ______ energy level • Excited state: an atom, having _____ E, jumps its electron(s) to a higher E level. The electron must jump completely from one level to another.

• De-excitation: after a short time the electron ______________ and _____ a photon (packet of E) equivalent to the energy difference between the 2 steps. The photon will produce a spectral line with a discrete wavelength (color) associated with it. • Continuous spectrum: all wavelengths are present (i. e. sunlight) • Emission (bright line) spectrum: limited # of specific bright lines that are produced by pass the light emitted by an excited atom through a prism

• Absorption (dark line) spectrum: light emitted by an excited atom passes through a substance that filters out certain wavelengths and thus produces a spectrum with missing (dark) lines. • Bright lines of an emission spectrum are ___________ as the dark lines of an absorption spectrum for a given element.

Bohr Model of the Atom, 1913 • Studied the absorption of light by ______ • Absorption of light at definite wavelengths corresponds to the definite changes in the E of the ________ • Electrons can circle the nucleus at _____________ distances…only in allowed paths, or orbits (Satellite model) • Energy of an electron is ______ when it is in orbits farther away from the nucleus • His calculated energy values agreed with the observed spectral lines for hydrogen • Model did NOT work when applied to multi-electron elements

Duality of light • Proposed by Einstein after studying the works of Planck and Bohr • Light has been traditionally thought of as a _____ (it has freq. & wavelength) • But now. . light can also be thought of as a ______ of particles called photons • photons: particle of EM radiation having _______ mass and carrying a _______ of energy • Explained photoelectric effect: EM radiation is absorbed only in whole numbers of photons

4 -2 The Quantum Model of the Atom

De Broglie, 1924 • If light could behave as both a wave and a particle, then could an electron (a particle) also behave as both a particle and a wave ? ? ? ? • He said “_____” because…. – Since electrons could only exist at specific energies, and E can be equated to frequency (E = hν), they have wave properties – And electrons can be “focused” like light (electron microscopes) – particle nature of electrons had already been confirmed (cathode rays, oil drop exper, etc. )

• Electrons have a dual wave-particle nature

Heisenberg Uncertainty Principle • It is impossible to determine simultaneously both the _____ and _____ of an electron or any other particle

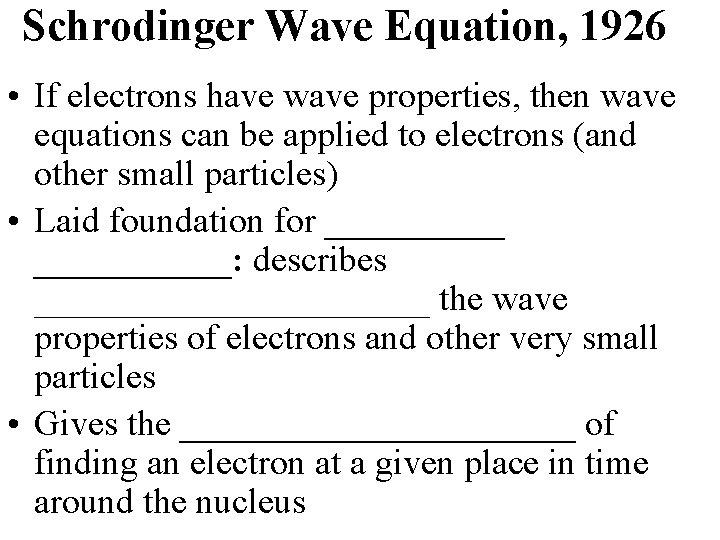
Schrodinger Wave Equation, 1926 • If electrons have wave properties, then wave equations can be applied to electrons (and other small particles) • Laid foundation for ___________: describes ___________ the wave properties of electrons and other very small particles • Gives the ___________ of finding an electron at a given place in time around the nucleus

Quantum Theory (con’t) • Electrons do NOT travel around the nucleus like planets around the sun • Electrons exist in certain regions called _______: a 3 -D region around the nucleus that indicates the _____ location of an electron

Quantum Numbers • Specify the properties of atomic orbitals and the properties of electrons in orbitals • There are _____ quantum numbers…. the first three of which come from solutions to Schrodinger’s wave equation

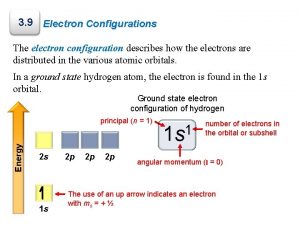
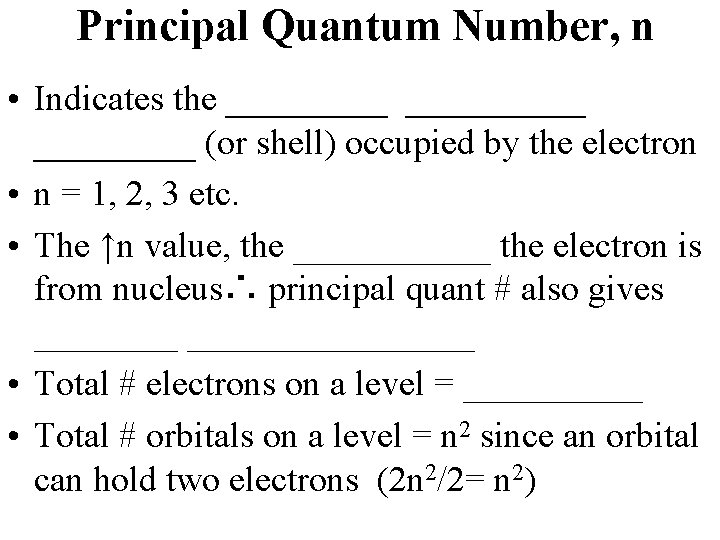
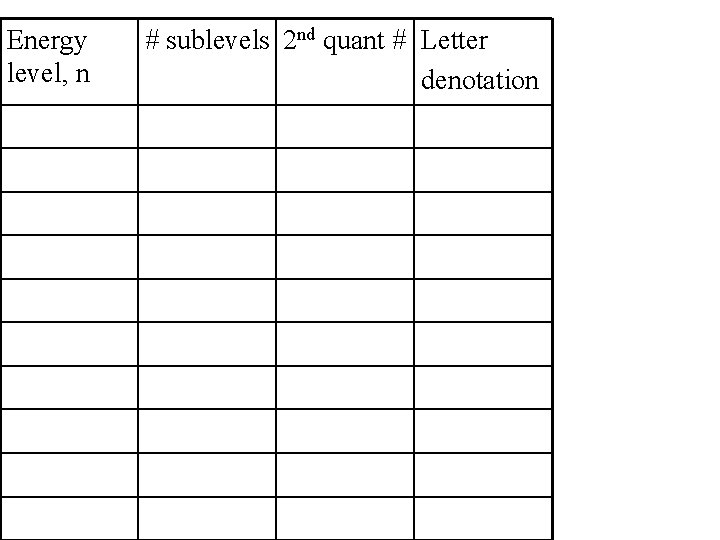
Principal Quantum Number, n • Indicates the __________ (or shell) occupied by the electron • n = 1, 2, 3 etc. • The ↑n value, the ______ the electron is from nucleus∴ principal quant # also gives ____________ • Total # electrons on a level = _____ • Total # orbitals on a level = n 2 since an orbital can hold two electrons (2 n 2/2= n 2)

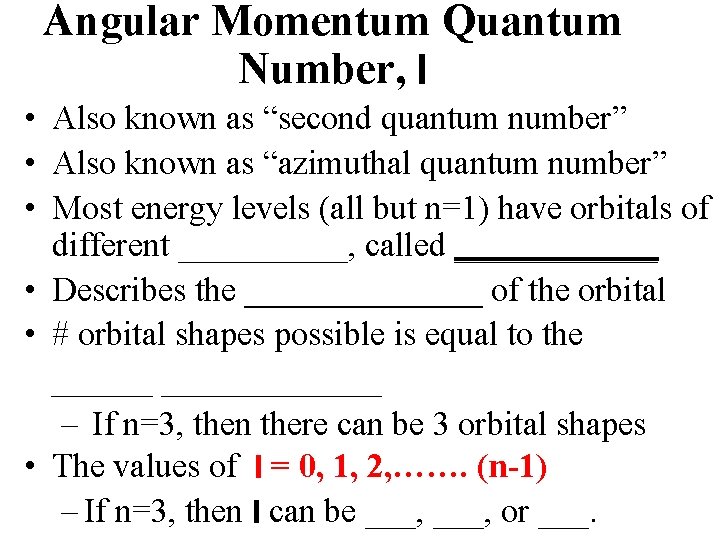
Angular Momentum Quantum Number, l • Also known as “second quantum number” • Also known as “azimuthal quantum number” • Most energy levels (all but n=1) have orbitals of different _____, called ______ • Describes the _______ of the orbital • # orbital shapes possible is equal to the _____________ – If n=3, then there can be 3 orbital shapes • The values of l = 0, 1, 2, ……. (n-1) – If n=3, then l can be ___, or ___.

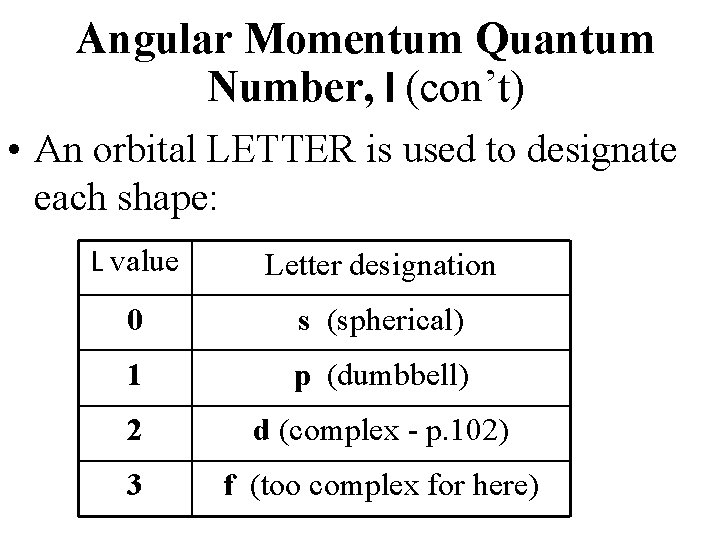
Angular Momentum Quantum Number, l (con’t) • An orbital LETTER is used to designate each shape: L value Letter designation 0 s (spherical) 1 p (dumbbell) 2 d (complex - p. 102) 3 f (too complex for here)

Energy level, n # sublevels 2 nd quant # Letter denotation

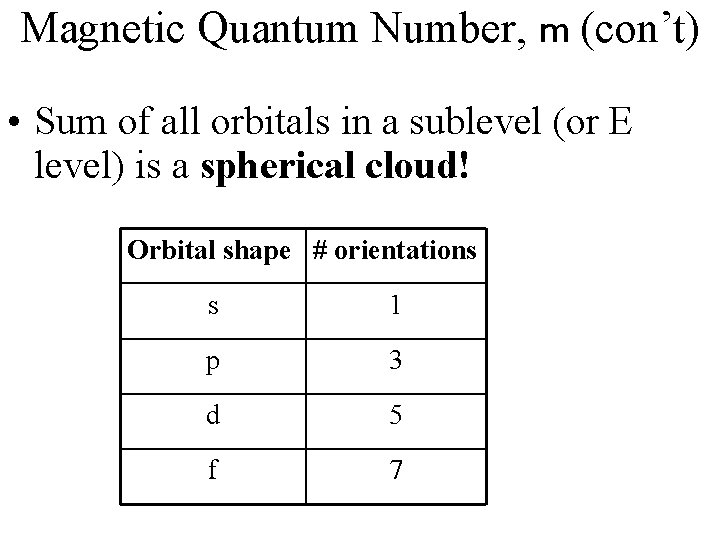
Magnetic Quantum Number, ml • also known as the 3 rd quantum number • indicates the orientation of an orbital around the nucleus • = (2 l + 1)

Magnetic Quantum Number, m (con’t) • Sum of all orbitals in a sublevel (or E level) is a spherical cloud! Orbital shape # orientations s 1 p 3 d 5 f 7

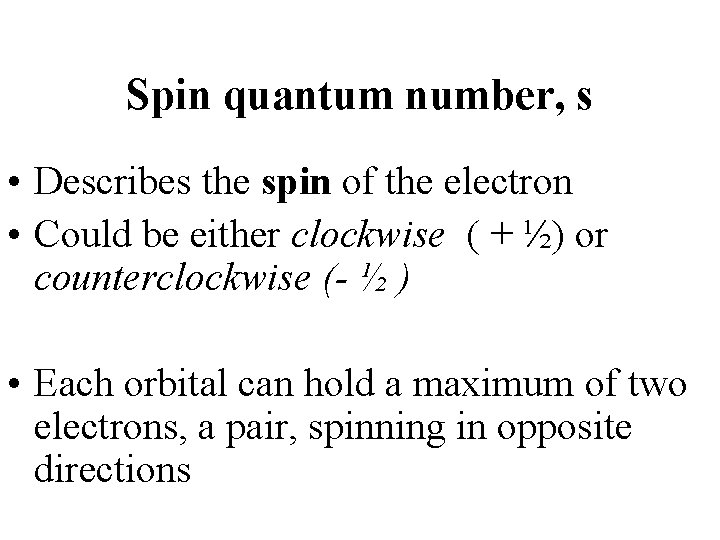
Spin quantum number, s • Describes the spin of the electron • Could be either clockwise ( + ½) or counterclockwise (- ½ ) • Each orbital can hold a maximum of two electrons, a pair, spinning in opposite directions

4 -3 ELECTRON CONFIGURATIONS

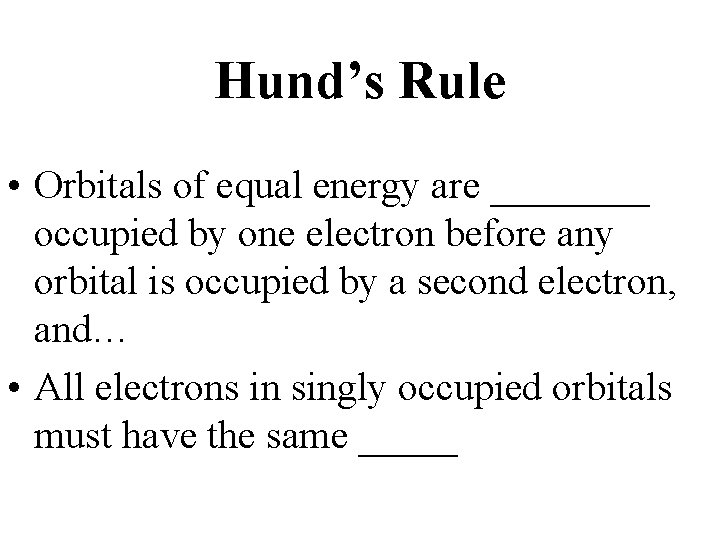
Hund’s Rule • Orbitals of equal energy are ____ occupied by one electron before any orbital is occupied by a second electron, and… • All electrons in singly occupied orbitals must have the same _____

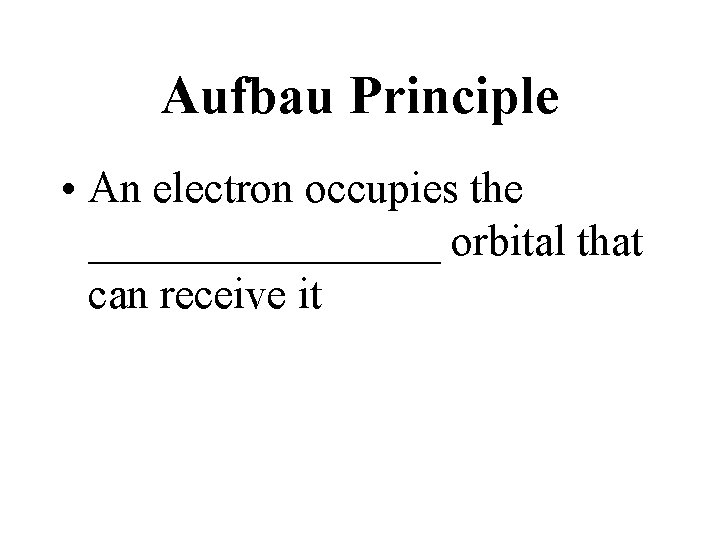
Aufbau Principle • An electron occupies the ________ orbital that can receive it

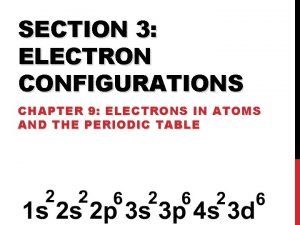
Noble Gas Notation • What is the noble gas electron notation for calcium? • 1 s 22 p 63 s 23 p 64 s 2 • What is the noble gas config for S? • 1 s 22 p 63 s 23 p 4

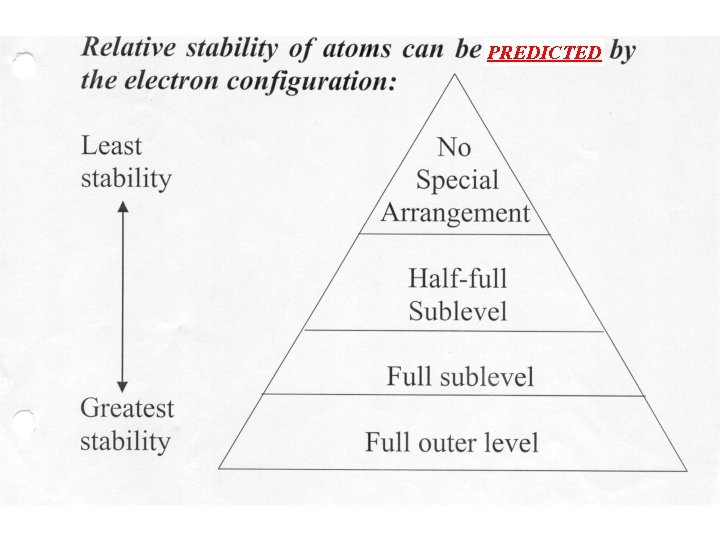
PREDICTED
 Chapter 4 arrangement of electrons in atoms test
Chapter 4 arrangement of electrons in atoms test Chapter 5 review arrangement of electrons in atoms
Chapter 5 review arrangement of electrons in atoms Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Chapter 5 electrons in atoms
Chapter 5 electrons in atoms Light light light chapter 23
Light light light chapter 23 Light light light chapter 22
Light light light chapter 22 Chapter 22
Chapter 22 Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Regents periodic table
Regents periodic table Atoms with 4 valence electrons
Atoms with 4 valence electrons Diamagnetic elements
Diamagnetic elements How to find the neutrons of an element
How to find the neutrons of an element Lowest allowable energy state of an atom
Lowest allowable energy state of an atom Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Aluminum chloride charge
Aluminum chloride charge How do chemists model the valence electrons of metal atoms?
How do chemists model the valence electrons of metal atoms? Metallic bond formula
Metallic bond formula Electrons in atoms section 3 electron configuration
Electrons in atoms section 3 electron configuration 5 electrons in atoms
5 electrons in atoms Unstable arrangement of atoms
Unstable arrangement of atoms Arrangement of electrons
Arrangement of electrons Electron configuration
Electron configuration Orbital diagram for ca
Orbital diagram for ca Electrons and light pogil activity 5-1
Electrons and light pogil activity 5-1 The arrangement of opposite elements (ex. light vs. dark)
The arrangement of opposite elements (ex. light vs. dark) Chapter 4 section 2 the structure of atoms
Chapter 4 section 2 the structure of atoms Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 electronic structure of atoms answers
Chapter 6 electronic structure of atoms answers Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Energy quanta
Energy quanta Chapter 3 atoms the building blocks of matter
Chapter 3 atoms the building blocks of matter Chapter 3 atoms the building blocks of matter
Chapter 3 atoms the building blocks of matter