Mole Notes 1 Atomic Mass Unit amu atomic






- Slides: 22

Mole Notes

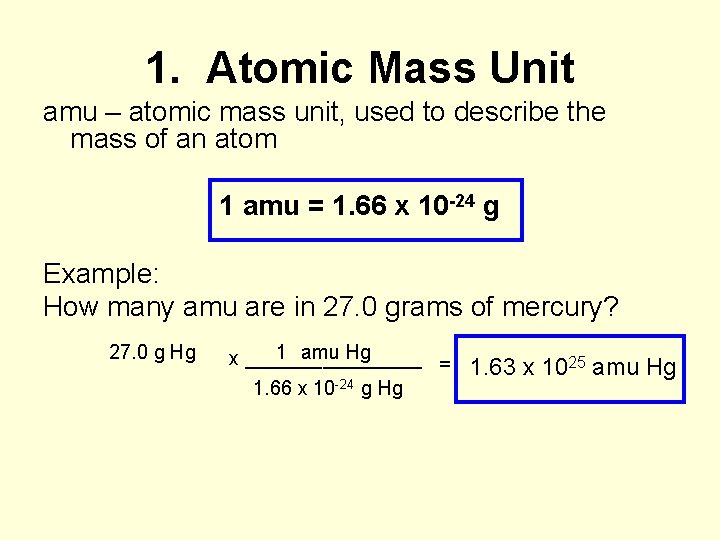
1. Atomic Mass Unit amu – atomic mass unit, used to describe the mass of an atom 1 amu = 1. 66 x 10 -24 g Example: How many amu are in 27. 0 grams of mercury? 27. 0 g Hg 1 amu Hg x ________ = 1. 66 x 10 -24 g Hg 1. 63 x 1025 amu Hg

Learning Check • How many grams are in 1. 73 x 1025 atomic mass units of silver?

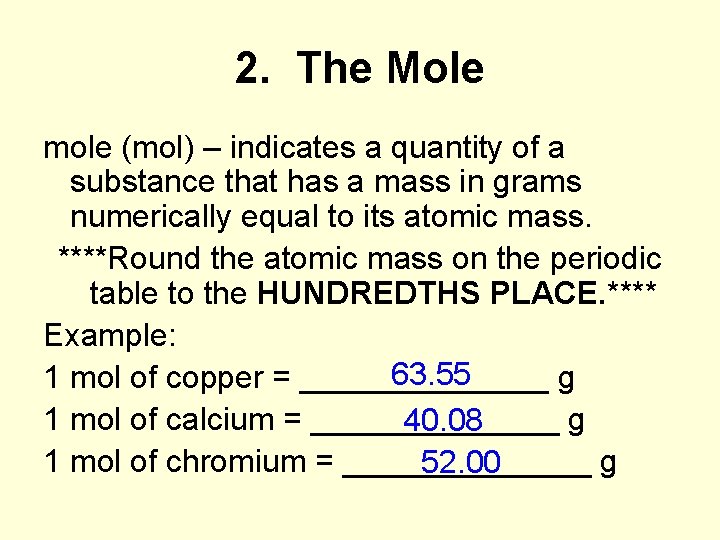
2. The Mole mole (mol) – indicates a quantity of a substance that has a mass in grams numerically equal to its atomic mass. ****Round the atomic mass on the periodic table to the HUNDREDTHS PLACE. **** Example: 63. 55 1 mol of copper = _______ g 1 mol of calcium = _______ g 40. 08 1 mol of chromium = _______ g 52. 00

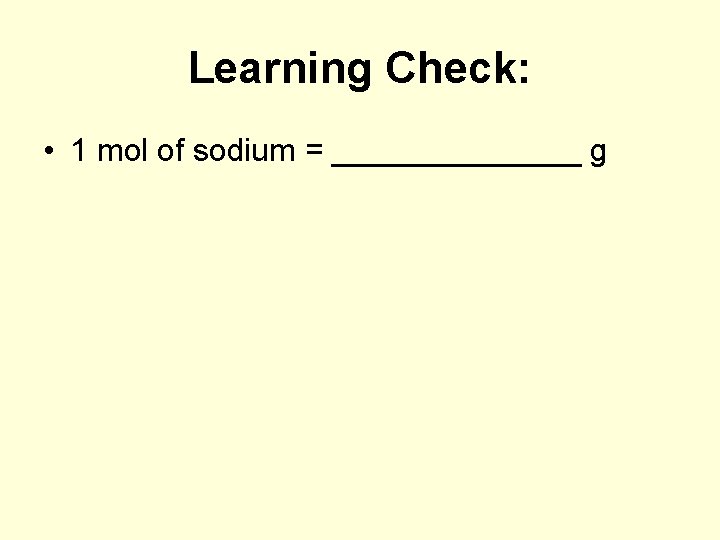
Learning Check: • 1 mol of sodium = _______ g

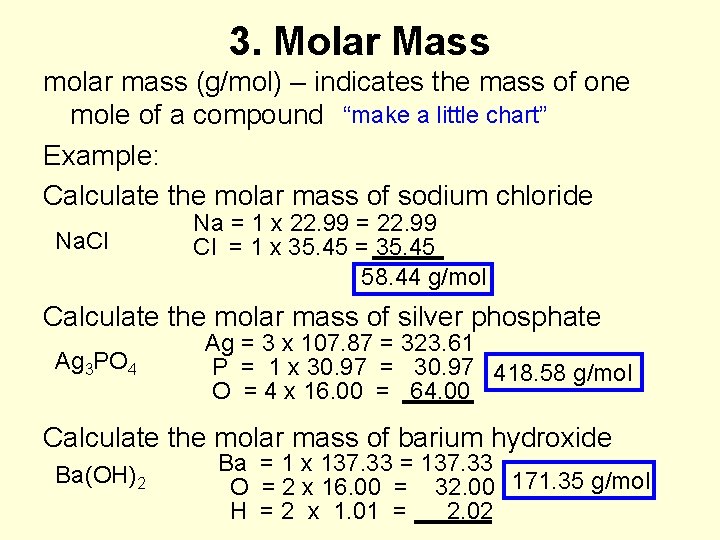
3. Molar Mass molar mass (g/mol) – indicates the mass of one mole of a compound “make a little chart” Example: Calculate the molar mass of sodium chloride Na. Cl Na = 1 x 22. 99 = 22. 99 Cl = 1 x 35. 45 = 35. 45 58. 44 g/mol Calculate the molar mass of silver phosphate Ag 3 PO 4 Ag = 3 x 107. 87 = 323. 61 P = 1 x 30. 97 = 30. 97 418. 58 g/mol O = 4 x 16. 00 = 64. 00 Calculate the molar mass of barium hydroxide Ba(OH)2 Ba = 1 x 137. 33 = 137. 33 O = 2 x 16. 00 = 32. 00 171. 35 g/mol H = 2 x 1. 01 = 2. 02

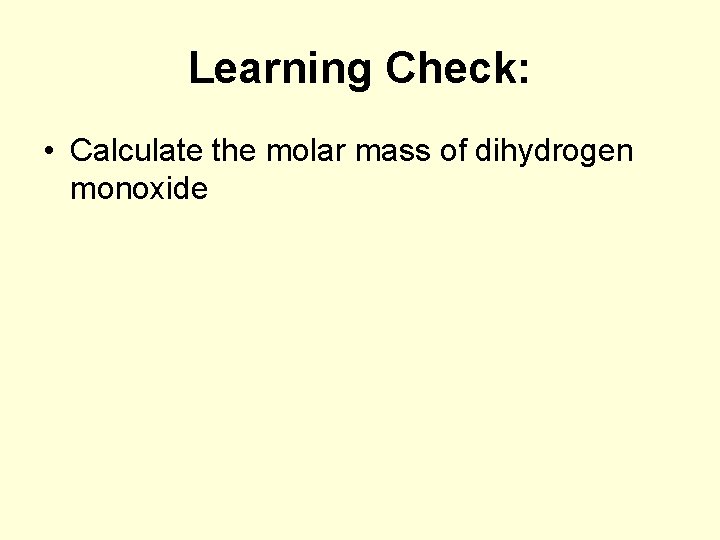
Learning Check: • Calculate the molar mass of dihydrogen monoxide

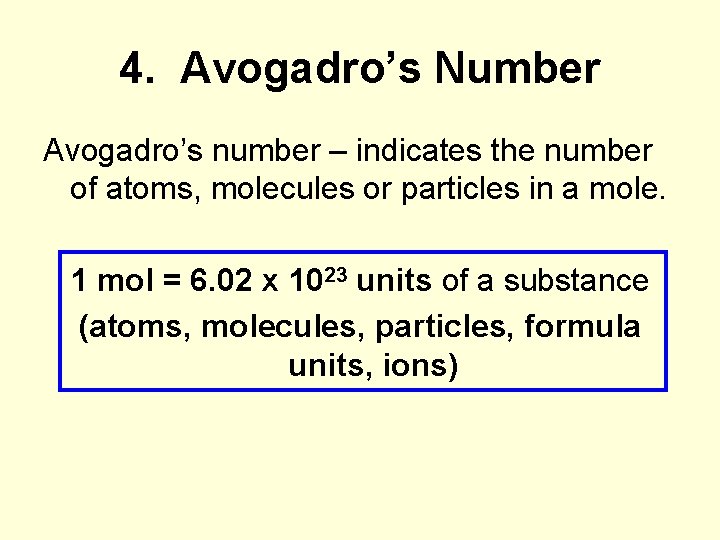
4. Avogadro’s Number Avogadro’s number – indicates the number of atoms, molecules or particles in a mole. 1 mol = 6. 02 x 1023 units of a substance (atoms, molecules, particles, formula units, ions)

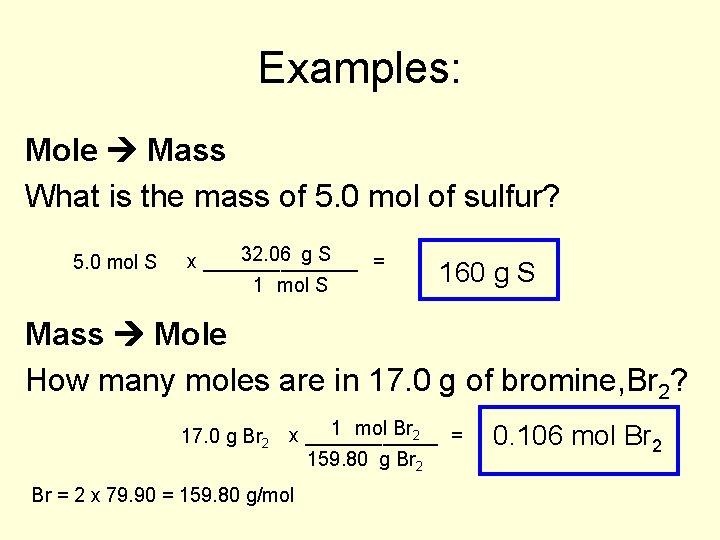
Examples: Mole Mass What is the mass of 5. 0 mol of sulfur? 5. 0 mol S 32. 06 g S x _______ = 1 mol S 160 g S Mass Mole How many moles are in 17. 0 g of bromine, Br 2? 1 mol Br 2 = 17. 0 g Br 2 x ______ 159. 80 g Br 2 Br = 2 x 79. 90 = 159. 80 g/mol 0. 106 mol Br 2

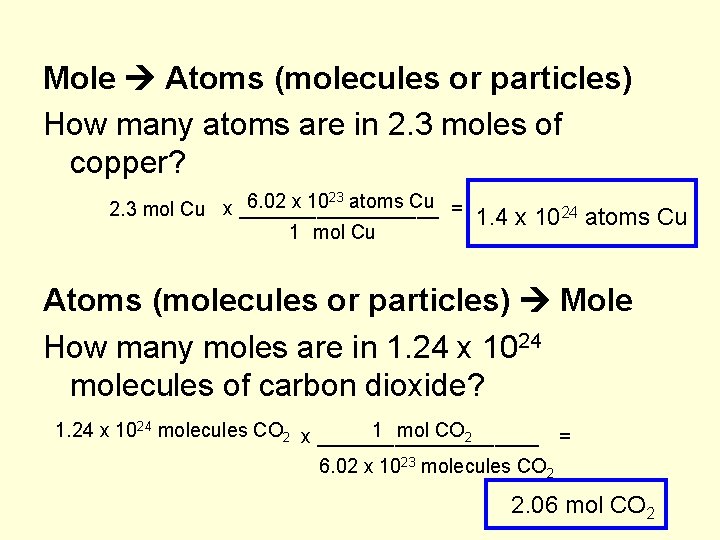
Mole Atoms (molecules or particles) How many atoms are in 2. 3 moles of copper? 23 atoms Cu 6. 02 x 10 x _________ = 2. 3 mol Cu 1. 4 x 1024 atoms Cu Atoms (molecules or particles) Mole How many moles are in 1. 24 x 1024 molecules of carbon dioxide? 1. 24 x 1024 molecules CO 2 x __________ 1 mol CO 2 = 6. 02 x 1023 molecules CO 2 2. 06 mol CO 2

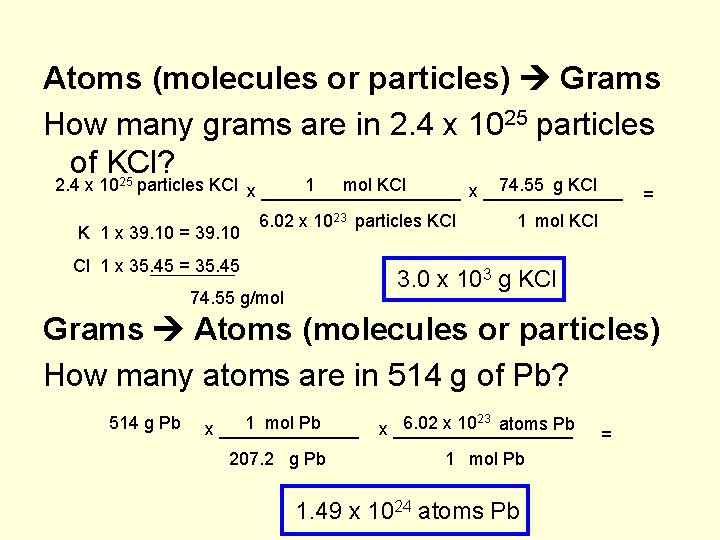
Atoms (molecules or particles) Grams How many grams are in 2. 4 x 1025 particles of KCl? 2. 4 x 1025 particles KCl x __________ 1 mol KCl 74. 55 g KCl x _______ K 1 x 39. 10 = 39. 10 6. 02 x 1023 particles KCl Cl 1 x 35. 45 = 1 mol KCl 3. 0 x 103 g KCl 74. 55 g/mol Grams Atoms (molecules or particles) How many atoms are in 514 g of Pb? 514 g Pb 1 mol Pb x _______ 207. 2 g Pb 6. 02 x 1023 atoms Pb x _________ 1 mol Pb 1. 49 x 1024 atoms Pb =

Learning Check: • How many particles are in 8. 75 g of silver nitrate?

Mole Lab

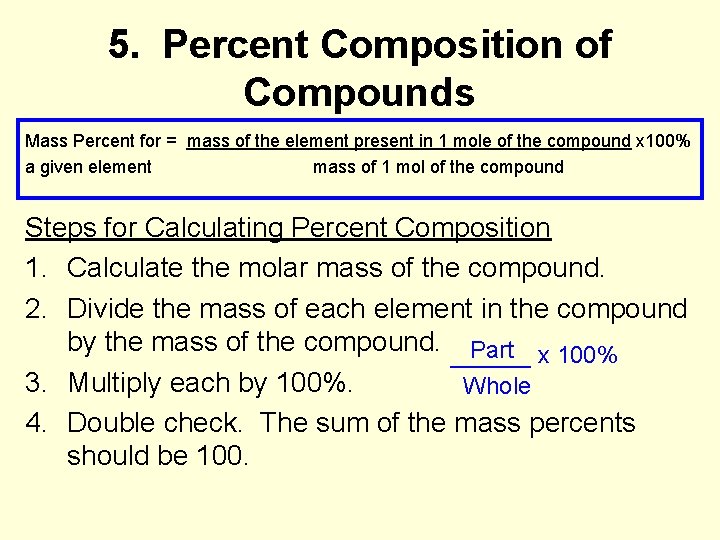
5. Percent Composition of Compounds Mass Percent for = mass of the element present in 1 mole of the compound x 100% a given element mass of 1 mol of the compound Steps for Calculating Percent Composition 1. Calculate the molar mass of the compound. 2. Divide the mass of each element in the compound by the mass of the compound. ______ Part x 100% 3. Multiply each by 100%. Whole 4. Double check. The sum of the mass percents should be 100.

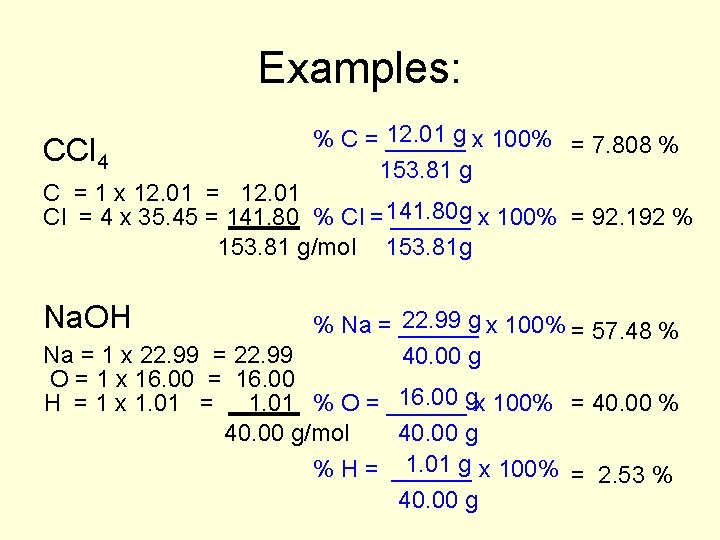
Examples: CCl 4 12. 01 g x 100% = 7. 808 % % C = ______ 153. 81 g C = 1 x 12. 01 = 12. 01 Cl = 4 x 35. 45 = 141. 80 % Cl =141. 80 g ______ x 100% = 92. 192 % 153. 81 g/mol 153. 81 g Na. OH 22. 99 g x 100% = 57. 48 % % Na = ______ Na = 1 x 22. 99 = 22. 99 40. 00 g O = 1 x 16. 00 = 16. 00 gx 100% = 40. 00 % H = 1 x 1. 01 = 1. 01 % O = ______ 40. 00 g/mol 40. 00 g 1. 01 g x 100% = 2. 53 % % H = ______ 40. 00 g

Learning Check: • Determine the percent composition for each element in dinitrogen pentoxide.

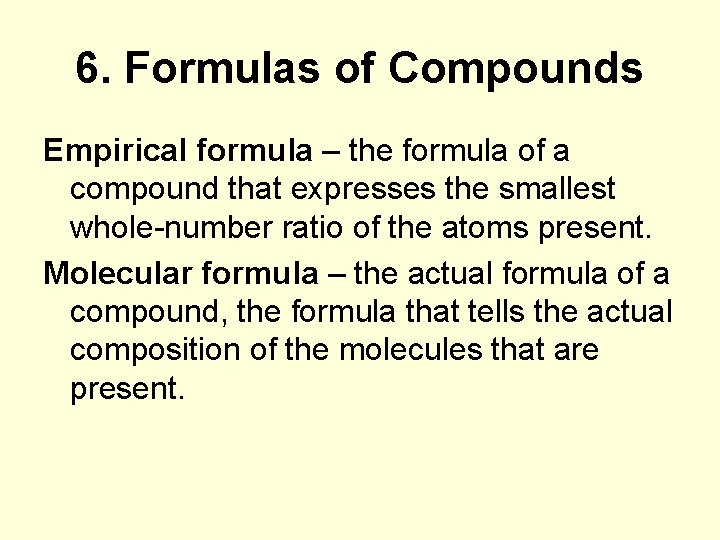
6. Formulas of Compounds Empirical formula – the formula of a compound that expresses the smallest whole-number ratio of the atoms present. Molecular formula – the actual formula of a compound, the formula that tells the actual composition of the molecules that are present.

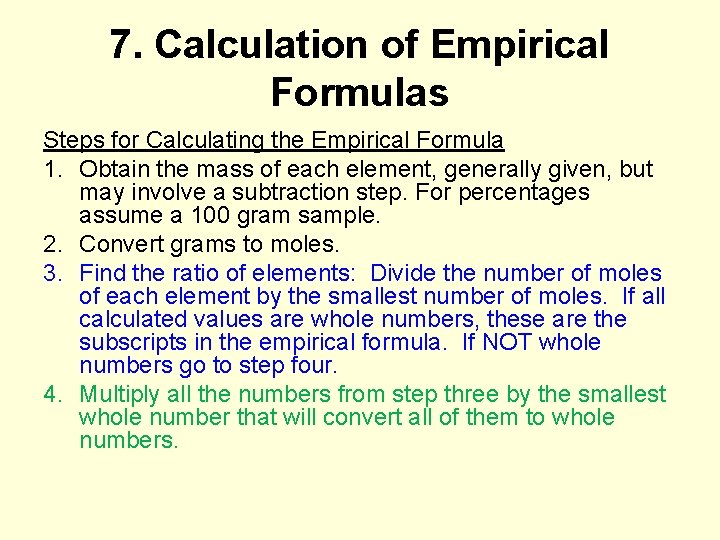
7. Calculation of Empirical Formulas Steps for Calculating the Empirical Formula 1. Obtain the mass of each element, generally given, but may involve a subtraction step. For percentages assume a 100 gram sample. 2. Convert grams to moles. 3. Find the ratio of elements: Divide the number of moles of each element by the smallest number of moles. If all calculated values are whole numbers, these are the subscripts in the empirical formula. If NOT whole numbers go to step four. 4. Multiply all the numbers from step three by the smallest whole number that will convert all of them to whole numbers.

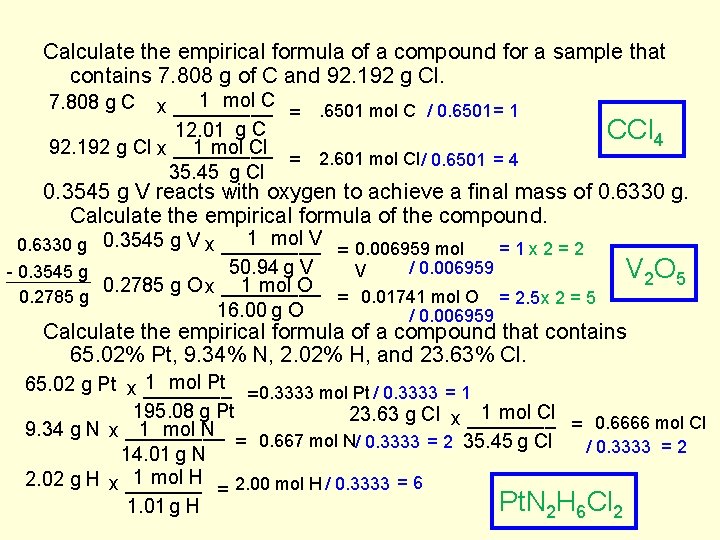
Calculate the empirical formula of a compound for a sample that contains 7. 808 g of C and 92. 192 g Cl. 1 mol C x _____ =. 6501 mol C / 0. 6501 = 1 12. 01 g C 1 mol Cl 92. 192 g Cl x _____ = 2. 601 mol Cl / 0. 6501 = 4 35. 45 g Cl 7. 808 g C CCl 4 0. 3545 g V reacts with oxygen to achieve a final mass of 0. 6330 g. Calculate the empirical formula of the compound. 1 mol V 0. 6330 g 0. 3545 g V x _____ =1 x 2=2 = 0. 006959 mol 50. 94 g V / 0. 006959 V - 0. 3545 g 1 mol O 0. 2785 g O x _____ 0. 2785 g = 0. 01741 mol O = 2. 5 x 2 = 5 16. 00 g O / 0. 006959 V 2 O 5 Calculate the empirical formula of a compound that contains 65. 02% Pt, 9. 34% N, 2. 02% H, and 23. 63% Cl. 1 mol Pt 65. 02 g Pt x ____ =0. 3333 mol Pt / 0. 3333 = 1 195. 08 g Pt 1 mol Cl 23. 63 g Cl x ____ = 0. 6666 mol Cl 1 mol N 9. 34 g N x _____ = 0. 667 mol N/ 0. 3333 = 2 35. 45 g Cl / 0. 3333 = 2 14. 01 g N 1 mol H 2. 02 g H x _______ = 2. 00 mol H / 0. 3333 = 6 Pt. N 2 H 6 Cl 2 1. 01 g H

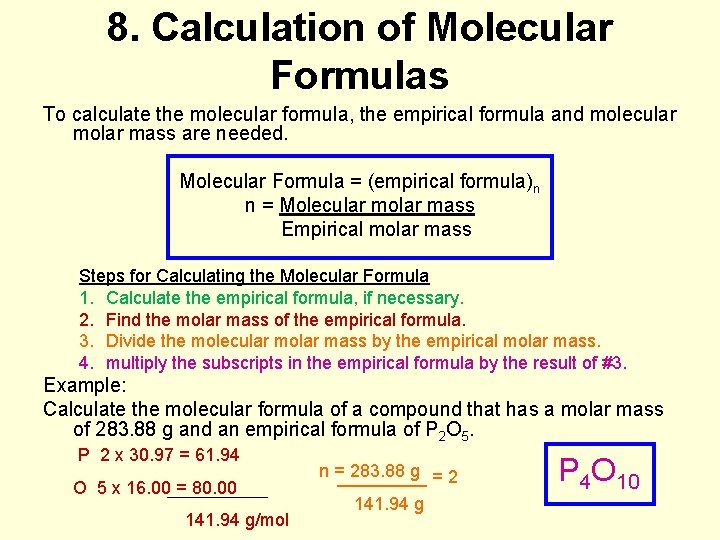
8. Calculation of Molecular Formulas To calculate the molecular formula, the empirical formula and molecular molar mass are needed. Molecular Formula = (empirical formula)n n = Molecular molar mass Empirical molar mass Steps for Calculating the Molecular Formula 1. Calculate the empirical formula, if necessary. 2. Find the molar mass of the empirical formula. 3. Divide the molecular molar mass by the empirical molar mass. 4. multiply the subscripts in the empirical formula by the result of #3. Example: Calculate the molecular formula of a compound that has a molar mass of 283. 88 g and an empirical formula of P 2 O 5. P 2 x 30. 97 = 61. 94 O 5 x 16. 00 = 80. 00 141. 94 g/mol n =_____ 283. 88 g = 2 141. 94 g P 4 O 10

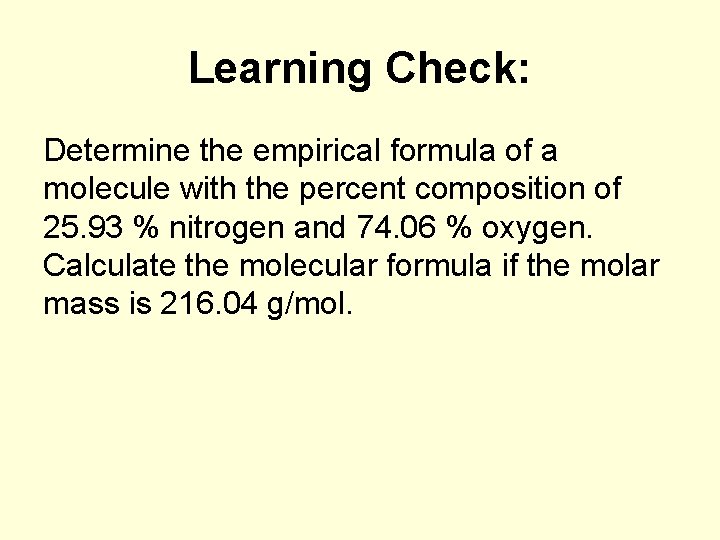
Learning Check: Determine the empirical formula of a molecule with the percent composition of 25. 93 % nitrogen and 74. 06 % oxygen. Calculate the molecular formula if the molar mass is 216. 04 g/mol.
