Moles MOLES Moles Atoms Moles Grams Copyright Sautter












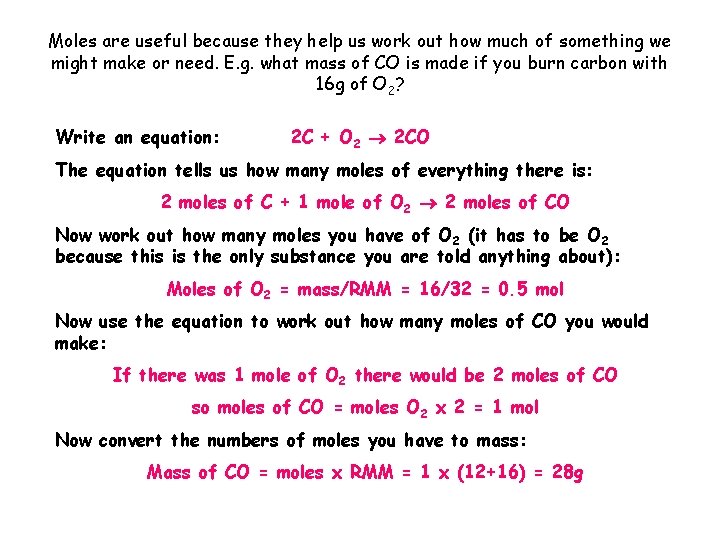





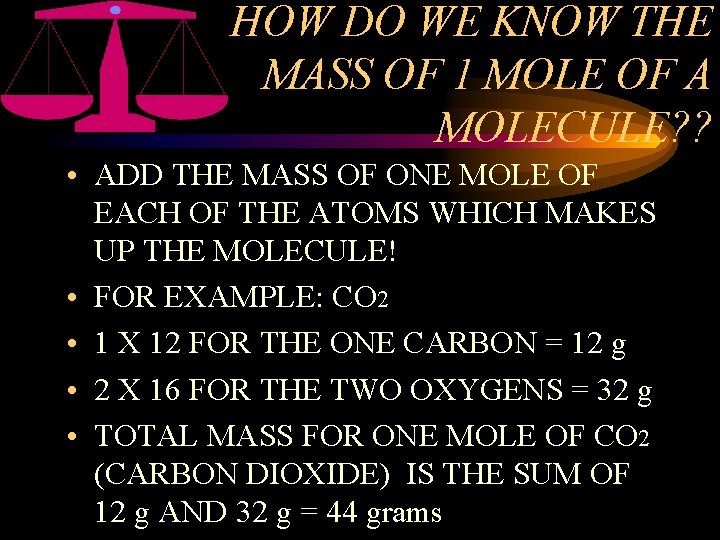



- Slides: 50

Moles MOLES Moles Atoms Moles Grams Copyright Sautter 2003

Introduction • How did you determine the number of M&Ms in the bag without actually counting?

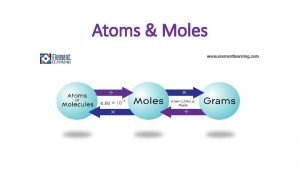
Atoms • Can you determine the number of atoms of an element even though you can not actually count them? • Yes… IF there is a conversion factor that allows you to convert between mass and number of atoms.

Key Terms • • • Relative atomic mass (RAM or Ar) Relative formula mass (RFM) Relative molecular mass (RMM or Mr) Avogadro’s number Mole

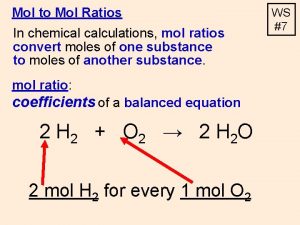
Moles Calculations By the end of the lesson you will be able to. . Rearrange the mole triangle to convert mass to moles and vice versa. Work out the formula of a substance if you know the percentage masses.

To start. . . • What is a mole (description)? • What is the value for a mole? • If we had a mole of bananas, how many bananas would we have? • If we had a mole of carbon atoms, how many atoms would we have? • What is the mass of a mole of carbon atoms?

What is a mole? • A mole is a name for a number just like the word dozen is a name for a number. • As you know, a dozen means the number 12. • A mole is a number too but a much, much bigger number. • A mole is 602, 000, 000, 000. In words it is six hundred and two hexillion ! • Why is a mole so big? Because it is used to count atoms and molecules which are very, very small, It takes lots of them just to be seen much less work with them. A speck of dusk contains trillions of atoms or more!



Mass, Moles and RAM (Mr) Mass Moles RAM

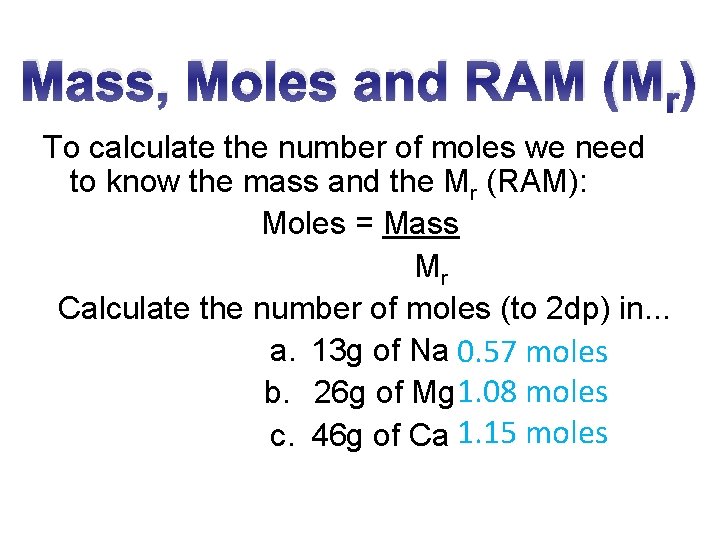
Mass, Moles and RAM (Mr) To calculate the number of moles we need to know the mass and the Mr (RAM): Moles = Mass Mr Calculate the number of moles (to 2 dp) in. . . a. 13 g of Na 0. 57 moles b. 26 g of Mg 1. 08 moles c. 46 g of Ca 1. 15 moles

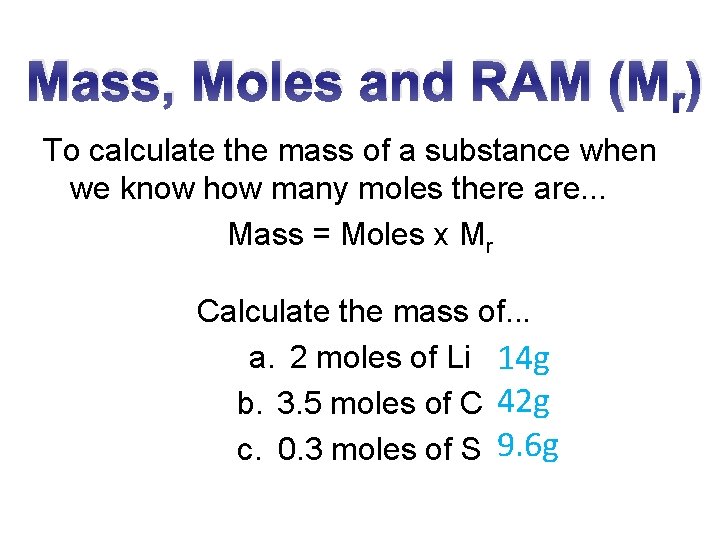
Mass, Moles and RAM (Mr) To calculate the mass of a substance when we know how many moles there are. . . Mass = Moles x Mr Calculate the mass of. . . a. 2 moles of Li 14 g b. 3. 5 moles of C 42 g c. 0. 3 moles of S 9. 6 g

Counting Atoms and Molecules • If atoms and molecules are so small and numerous, how can they be counted? ? • If atoms and molecules could be seen and all the people on the earth began counting the water molecules in our five teaspoons of water and if everyone counted day and night for his entire life, and if we added all their results together we would still not count them all !! • Counting atoms and molecules is difficult but we have an easy way to count them.

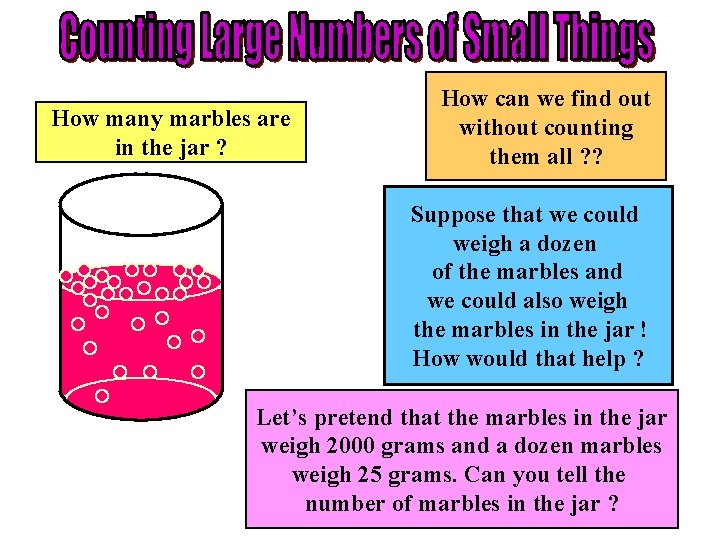
How many marbles are in the jar ? How can we find out without counting them all ? ? Suppose that we could weigh a dozen of the marbles and we could also weigh the marbles in the jar ! How would that help ? Let’s pretend that the marbles in the jar weigh 2000 grams and a dozen marbles weigh 25 grams. Can you tell the number of marbles in the jar ?

COUNTING LARGE NUMBERS OF SMALL THINGS • In our marble example, the marbles weighed 2000 grams and one dozen of the marbles weighed 25 grams. • Dividing 2000 grams (all the marbles) by 25 grams (the weight of one dozen) gives 80 dozen marbles. • Multiplying 80 times 12 we get 960 marbles in the jar without actually counting all of the marble ! • In the case of atoms or molecules the same idea can be used. If we know the weight of a number of a certain type of atom or molecule and the weight of the sample that we have we can then determine the number of atoms or molecules in the sample by division and multiplication.

COUNTING LARGE NUMBERS OF SMALL THINGS • In counting atoms and molecules we will not use a dozen as our counted quantity. A dozen atoms would be so small that they could never even be seen with traditional microscopes. • Our counted quantity will be one mole (6. 02 x 1023 *) atoms or molecules. Unlike our marble example we will not count out and weigh the reference sample. Luckily, this work has been done for us. • The Periodic Table of the Elements lists the weight (mass) of one mole of every element. We can find the weight of one mole of each element in grams. • * 6. 02 x 1023 = 602, 000, 000, 000 = 1 mole

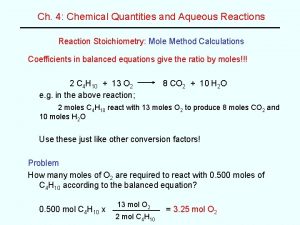
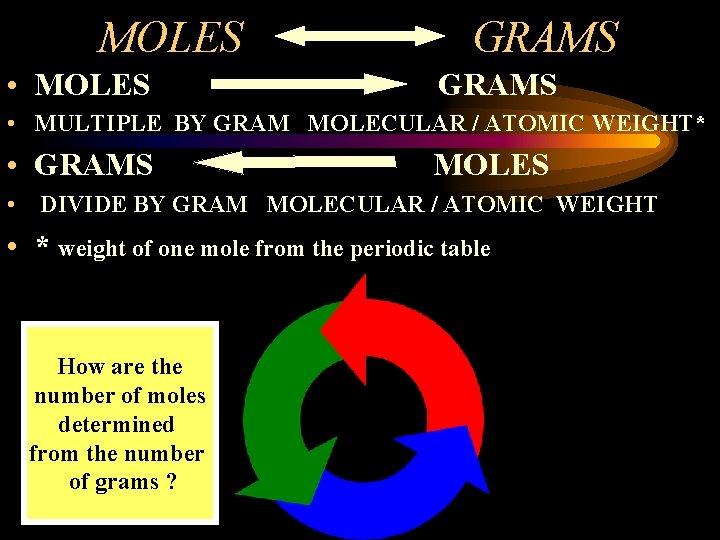
MOLES • MOLES GRAMS • MULTIPLE BY GRAM MOLECULAR / ATOMIC WEIGHT* • GRAMS MOLES • DIVIDE BY GRAM MOLECULAR / ATOMIC WEIGHT • * weight of one mole from the periodic table How are the number of moles determined from the number of grams ?

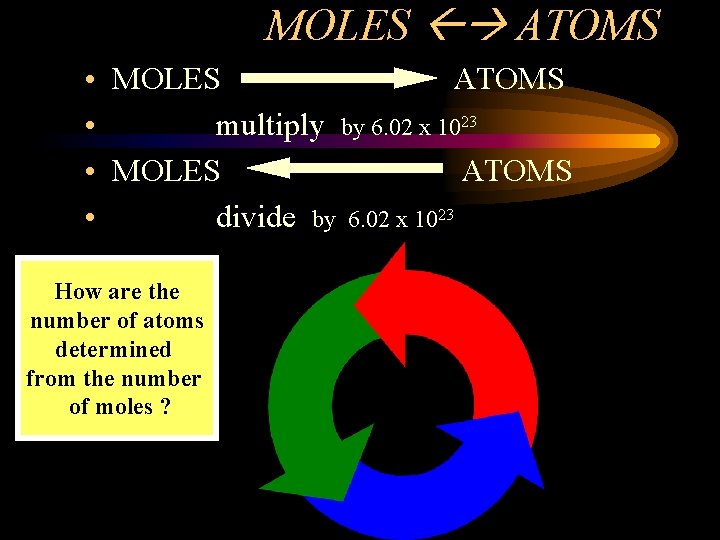
MOLES ATOMS • MOLES ATOMS • multiply by 6. 02 x 1023 • MOLES ATOMS • divide by 6. 02 x 1023 How are the number of atoms determined from the number of moles ?

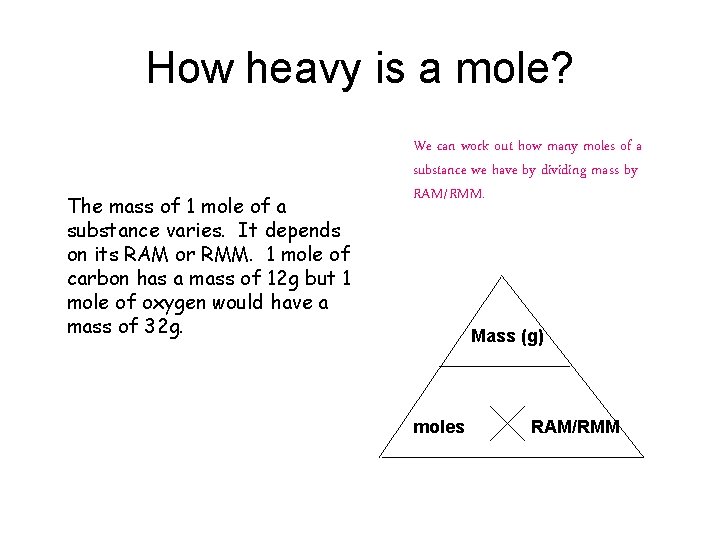
How heavy is a mole? The mass of 1 mole of a substance varies. It depends on its RAM or RMM. 1 mole of carbon has a mass of 12 g but 1 mole of oxygen would have a mass of 32 g. We can work out how many moles of a substance we have by dividing mass by RAM/RMM. Mass (g) moles RAM/RMM

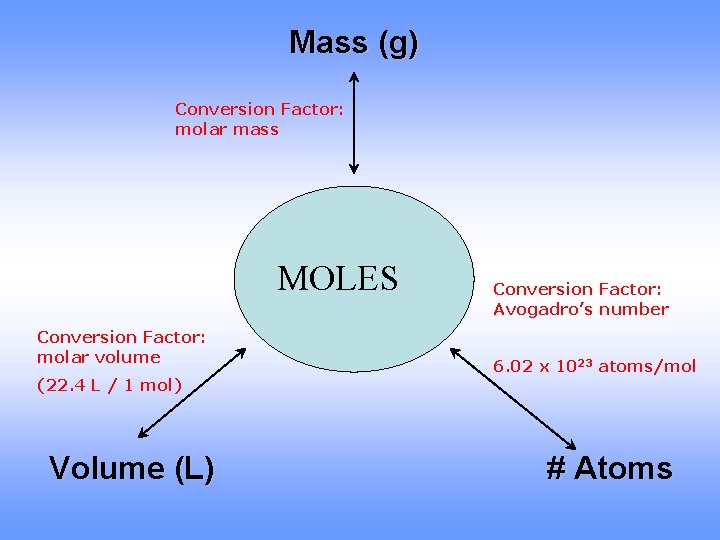
Mass (g) Conversion Factor: molar mass MOLES Conversion Factor: molar volume (22. 4 L / 1 mol) Volume (L) Conversion Factor: Avogadro’s number 6. 02 x 1023 atoms/mol # Atoms

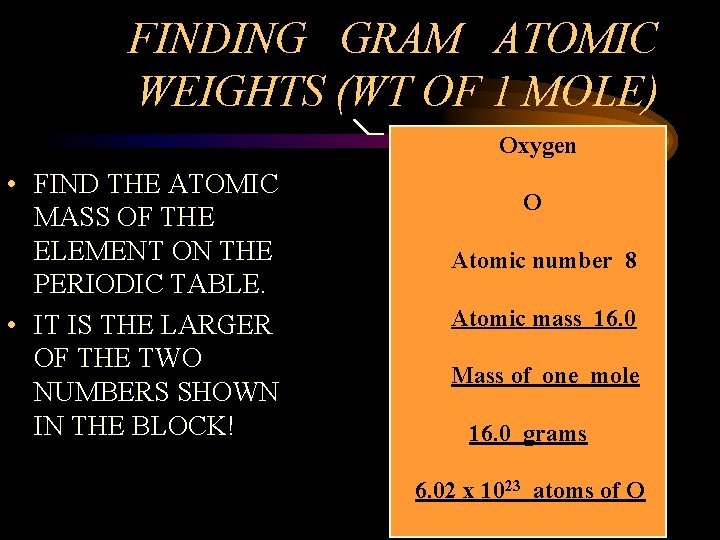
FINDING GRAM ATOMIC WEIGHTS (WT OF 1 MOLE) Oxygen • FIND THE ATOMIC MASS OF THE ELEMENT ON THE PERIODIC TABLE. • IT IS THE LARGER OF THE TWO NUMBERS SHOWN IN THE BLOCK! O Atomic number 8 Atomic mass 16. 0 Mass of one mole 16. 0 grams 6. 02 x 1023 atoms of O

Let’s try some problems with moles ! How many moles are contained in 36. 0 grams of oxygen? Solution: From the Periodic Table 36. 0 grams / 16. 0 grams per mole = 2. 25 moles 36. 0 grams contains 2. 25 moles of Oxygen Atoms

How many atoms are contained in 36. 0 grams of oxygen? Solution: 36. 0 grams / 16. 0 grams per mole = 2. 25 moles of O 2. 25 mole x (6. 02 x 1023 atoms per mole) = 1. 35 x 1024 atoms of oxygen

How many moles are contained in 3. 6 x 1024 atoms? • Solution: • (3. 6 x 1024 atoms) / (6. 02 x 1023) = 6. 0 moles • OF ANY KIND OF ATOMS !!

How many grams does 3. 6 x 1024 atoms of S “weigh” (mass)? • SOLUTION: • From the Periodic Table, Gram Atomic Mass of S is 32. 1 grams • (3. 6 x 1024) / (6. 02 x 1023) = 6. 0 moles of S • 6. 0 x 32. 1 = 192. 6 grams of Sulfur is the “weight” (mass) of 3. 6 x 1024 atoms of S!
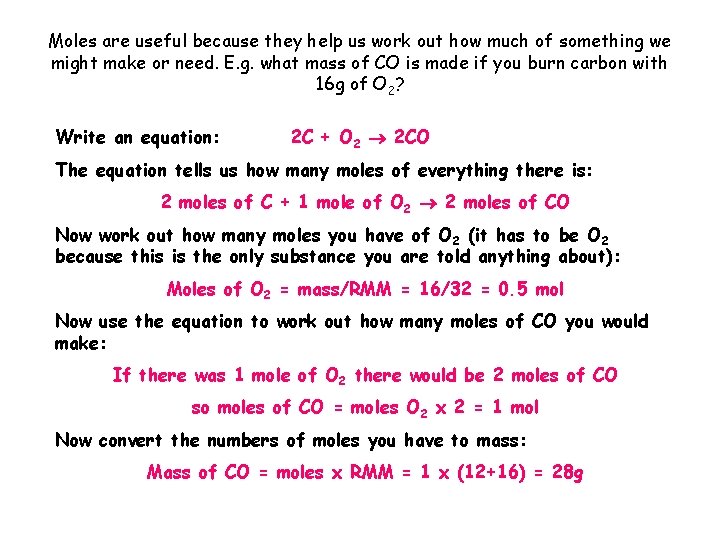
Moles are useful because they help us work out how much of something we might make or need. E. g. what mass of CO is made if you burn carbon with 16 g of O 2? Write an equation: 2 C + O 2 2 CO The equation tells us how many moles of everything there is: 2 moles of C + 1 mole of O 2 2 moles of CO Now work out how many moles you have of O 2 (it has to be O 2 because this is the only substance you are told anything about): Moles of O 2 = mass/RMM = 16/32 = 0. 5 mol Now use the equation to work out how many moles of CO you would make: If there was 1 mole of O 2 there would be 2 moles of CO so moles of CO = moles O 2 x 2 = 1 mol Now convert the numbers of moles you have to mass: Mass of CO = moles x RMM = 1 x (12+16) = 28 g

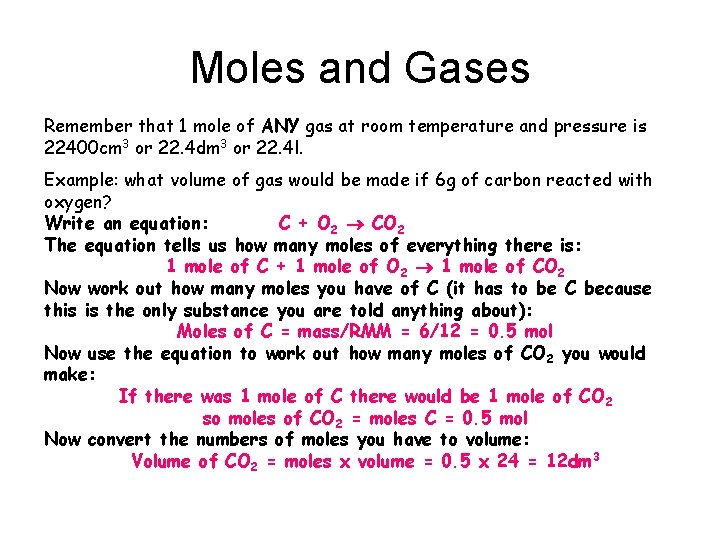
Moles and Gases Remember that 1 mole of ANY gas at room temperature and pressure is 22400 cm 3 or 22. 4 dm 3 or 22. 4 l. Example: what volume of gas would be made if 6 g of carbon reacted with oxygen? Write an equation: C + O 2 CO 2 The equation tells us how many moles of everything there is: 1 mole of C + 1 mole of O 2 1 mole of CO 2 Now work out how many moles you have of C (it has to be C because this is the only substance you are told anything about): Moles of C = mass/RMM = 6/12 = 0. 5 mol Now use the equation to work out how many moles of CO 2 you would make: If there was 1 mole of C there would be 1 mole of CO 2 so moles of CO 2 = moles C = 0. 5 mol Now convert the numbers of moles you have to volume: Volume of CO 2 = moles x volume = 0. 5 x 24 = 12 dm 3

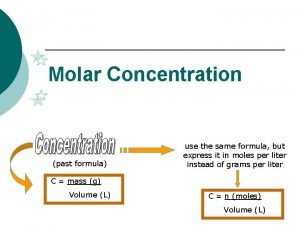
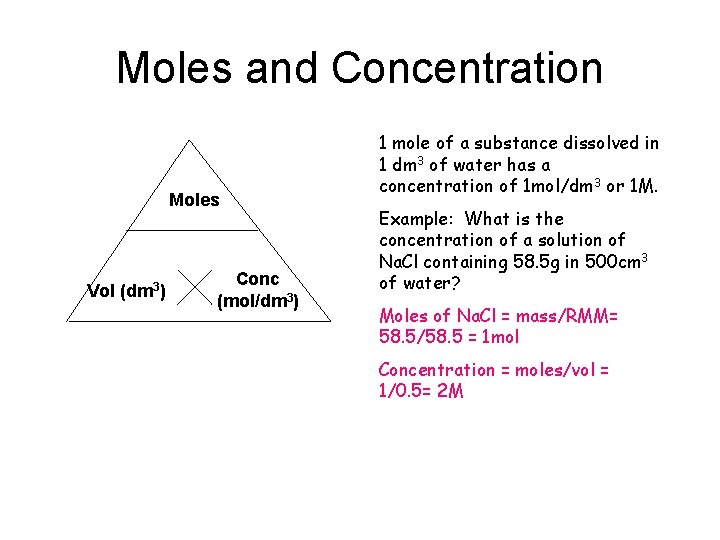
Moles and Concentration Moles Vol (dm 3) Conc (mol/dm 3) 1 mole of a substance dissolved in 1 dm 3 of water has a concentration of 1 mol/dm 3 or 1 M. Example: What is the concentration of a solution of Na. Cl containing 58. 5 g in 500 cm 3 of water? Moles of Na. Cl = mass/RMM= 58. 5/58. 5 = 1 mol Concentration = moles/vol = 1/0. 5= 2 M

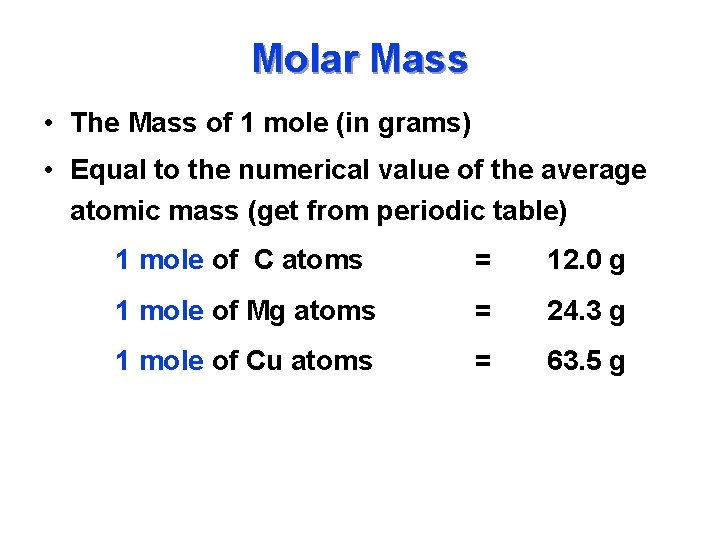
Molar Mass • The Mass of 1 mole (in grams) • Equal to the numerical value of the average atomic mass (get from periodic table) 1 mole of C atoms = 12. 0 g 1 mole of Mg atoms = 24. 3 g 1 mole of Cu atoms = 63. 5 g

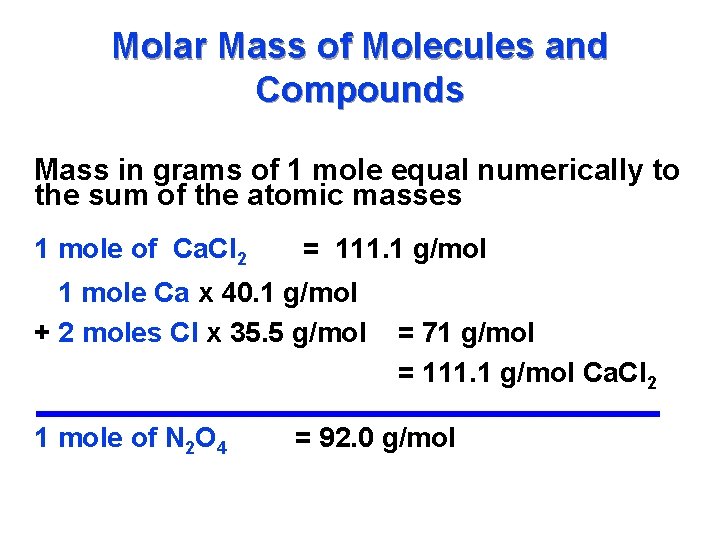
Molar Mass of Molecules and Compounds Mass in grams of 1 mole equal numerically to the sum of the atomic masses 1 mole of Ca. Cl 2 = 111. 1 g/mol 1 mole Ca x 40. 1 g/mol + 2 moles Cl x 35. 5 g/mol 1 mole of N 2 O 4 = 71 g/mol = 111. 1 g/mol Ca. Cl 2 = 92. 0 g/mol

Converting Moles and Grams Aluminum is often used for the structure of light-weight bicycle frames. How many grams of Al are in 3. 00 moles of Al? 3. 00 moles Al ? g Al

Learning Check! The artificial sweetener aspartame (Nutra-Sweet) formula C 14 H 18 N 2 O 5 is used to sweeten diet foods, coffee and soft drinks. How many moles of aspartame are present in 225 g of aspartame?

Calculations molar mass Grams Avogadro’s number Moles particles Everything must go through Moles!!!

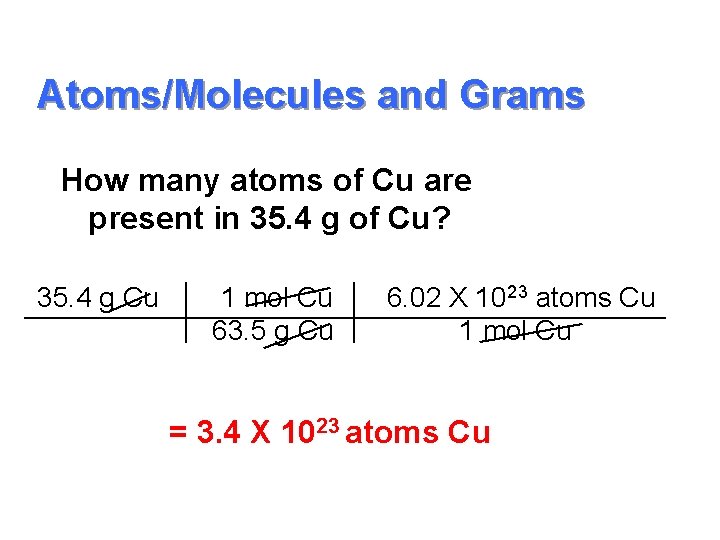
Atoms/Molecules and Grams How many atoms of Cu are present in 35. 4 g of Cu? 35. 4 g Cu 1 mol Cu 63. 5 g Cu 6. 02 X 1023 atoms Cu 1 mol Cu = 3. 4 X 1023 atoms Cu

Learning Check! How many atoms of K are present in 78. 4 g of K?

Learning Check! What is the mass (in grams) of 1. 20 X 1024 molecules of glucose (C 6 H 12 O 6)?

Percent Composition • Used to describe the make up of a compound by mass percentage • Compare the mass of each element present in 1 mole to the total mass of the compound

Sample Problem • Penicillin F has the formula C 14 H 20 N 2 SO 4 What is the mass percent of each element?

Empirical vs Molecular Formula • Molecular formula = (empirical formula)n – Where n is an integer • Assume a 100 g of the sample – Then each % will also be the mass • Determine the number of moles • Find ratio by using the smallest amount – Whole numbers represent subscripits

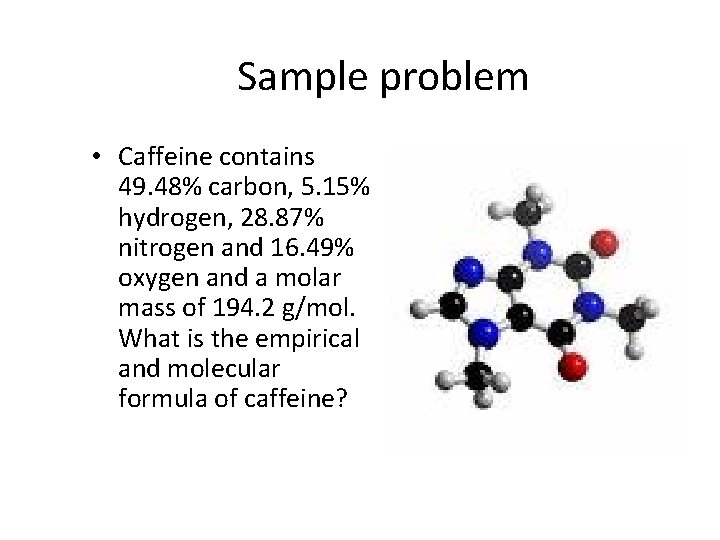
Sample problem • Caffeine contains 49. 48% carbon, 5. 15% hydrogen, 28. 87% nitrogen and 16. 49% oxygen and a molar mass of 194. 2 g/mol. What is the empirical and molecular formula of caffeine?

To obtain an Empirical Formula 1. Determine the mass in grams of each element present, if necessary. 2. Calculate the number of moles of each element. 3. Divide each by the smallest number of moles to obtain the simplest whole number ratio. 4. If whole numbers are not obtained* in step 3), multiply through by the smallest number that will give all whole numbers * Be careful! Do not round off numbers prematurely

Calculation of the Molecular Formula A compound has an empirical formula of NO 2. The colourless liquid, used in rocket engines has a molar mass of 92. 0 g/mole. What is the molecular formula of this substance?

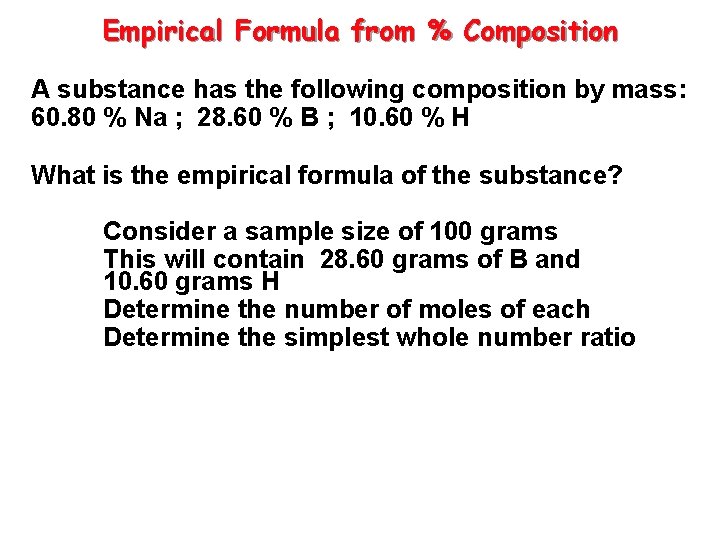
Empirical Formula from % Composition A substance has the following composition by mass: 60. 80 % Na ; 28. 60 % B ; 10. 60 % H What is the empirical formula of the substance? Consider a sample size of 100 grams This will contain 28. 60 grams of B and 10. 60 grams H Determine the number of moles of each Determine the simplest whole number ratio

AVOGADRO’S NUMBER! WHAT IS IT? ? • ONE MOLE IS 6. 02 x 1023 of ANYTHING just as ONE DOZEN 12 of ANYTHING! • This NUMBER (6. 02 x 1023) is called Avogadro’s Number!!

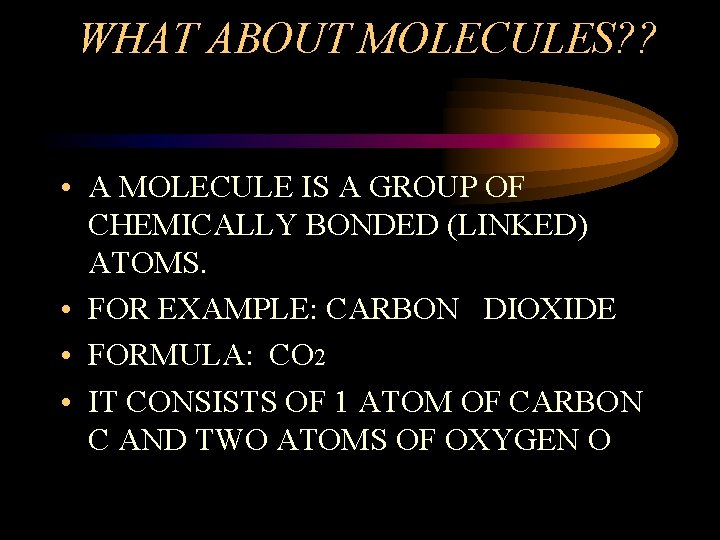
WHAT ABOUT MOLECULES? ? • A MOLECULE IS A GROUP OF CHEMICALLY BONDED (LINKED) ATOMS. • FOR EXAMPLE: CARBON DIOXIDE • FORMULA: CO 2 • IT CONSISTS OF 1 ATOM OF CARBON C AND TWO ATOMS OF OXYGEN O
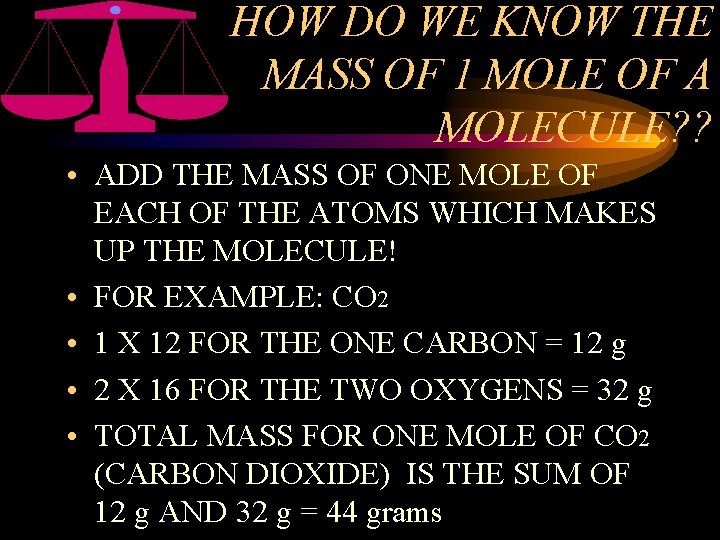
HOW DO WE KNOW THE MASS OF 1 MOLE OF A MOLECULE? ? • ADD THE MASS OF ONE MOLE OF EACH OF THE ATOMS WHICH MAKES UP THE MOLECULE! • FOR EXAMPLE: CO 2 • 1 X 12 FOR THE ONE CARBON = 12 g • 2 X 16 FOR THE TWO OXYGENS = 32 g • TOTAL MASS FOR ONE MOLE OF CO 2 (CARBON DIOXIDE) IS THE SUM OF 12 g AND 32 g = 44 grams

HOW DO WE FIND THE NUMBER OF MOLES, MASSES AND NUMBER OF MOLECULES THEN? ? • USE THE SAME STEPS AS WHEN DEALING WITH ATOMS EXCEPT WE NOW USE THE GRAM MOLECULAR WEIGHT (MASS) AS WE SHOWED IN THE PREVIOUS SLIDE!

FINDING THE NUMBER OF MOLES OF MOLECULES • HOW MANY MOLES OF CARBON DIOXIDE ARE CONTAINED 88. 0 GRAMS OF CO 2 ? • AS WE SAW EARLIER, 1 MOLE OF CO 2 HAS A MASS OF 44. 0 GRAMS. • 88. 0 grams / 44. 0 grams per mole = 2. 0 moles of CO 2 • HOW MANY MOLECULES ARE PRESENT? • 1 Mole = 6. 02 x 1023 molecules • 2. 0 mole x (6. 02 x 1023) = 12. 04 x 1023 CO 2 molecules • HOW MANY ATOMS ARE PRESENT? • Each CO 2 contains 1 carbon and 2 oxygen atoms, a total of 3 atoms in each molecule. • 12. 04 x 1023 molecules x 3 atoms per molecule = 36. 1 x 10 24 atoms

NOW IT’S YOUR TURN!! • TRY SOME PROBLEMS USING THE STEPS SHOWN HERE. • THE MORE YOU DO THE EASIER THEY GET!!!!

 Atoms to grams
Atoms to grams Periodic table of elements regents
Periodic table of elements regents Converting grams to moles
Converting grams to moles Mols to grams
Mols to grams How to dind moles
How to dind moles Mol to mol conversion
Mol to mol conversion Moles to grams
Moles to grams Moles to grams
Moles to grams Conversion from grams to moles
Conversion from grams to moles How to turn grams into moles
How to turn grams into moles Grams to moles examples
Grams to moles examples G to moles
G to moles Moles to grams
Moles to grams How to convert grams to mole
How to convert grams to mole Convert grams to moles
Convert grams to moles Dimensional analysis grams to moles
Dimensional analysis grams to moles You have 23 moles of tantalum (ta). how many grams is this
You have 23 moles of tantalum (ta). how many grams is this Grams to moles conversion
Grams to moles conversion Moles to grams
Moles to grams How to convert grams to moles
How to convert grams to moles Geams to moles
Geams to moles 16:00
16:00 Molecs
Molecs Atoms to mole
Atoms to mole Copyright
Copyright Atoms to moles
Atoms to moles If i place 3 moles of n2 and 4 moles of o2 in a 35l
If i place 3 moles of n2 and 4 moles of o2 in a 35l Items measured in grams
Items measured in grams Andrea grams
Andrea grams Liters to grams stoichiometry
Liters to grams stoichiometry The map below shows the location of chicxulub crater
The map below shows the location of chicxulub crater Papiercomputer
Papiercomputer Stand up sit down game
Stand up sit down game What weighs 1 gram
What weighs 1 gram A cookie contains 9 grams of fat
A cookie contains 9 grams of fat A person's weight is 154 pounds convert this to kilograms
A person's weight is 154 pounds convert this to kilograms Copy paste culture
Copy paste culture A solution is formed by dissolving 45 grams
A solution is formed by dissolving 45 grams 4 grams =
4 grams = Stoichiometry: mole-mole problems
Stoichiometry: mole-mole problems What does 27 grams of sugar look like
What does 27 grams of sugar look like Opp side equal angle
Opp side equal angle Timm grams
Timm grams Calculate the number of grams of al in 371g of al2o3
Calculate the number of grams of al in 371g of al2o3 Timm grams
Timm grams How to determine how much excess reactant is left over
How to determine how much excess reactant is left over Types of reactions
Types of reactions Parts of egg
Parts of egg Weight units
Weight units Limiting reactant
Limiting reactant Ppm water
Ppm water