Mol to Mol Ratios In chemical calculations mol




- Slides: 23

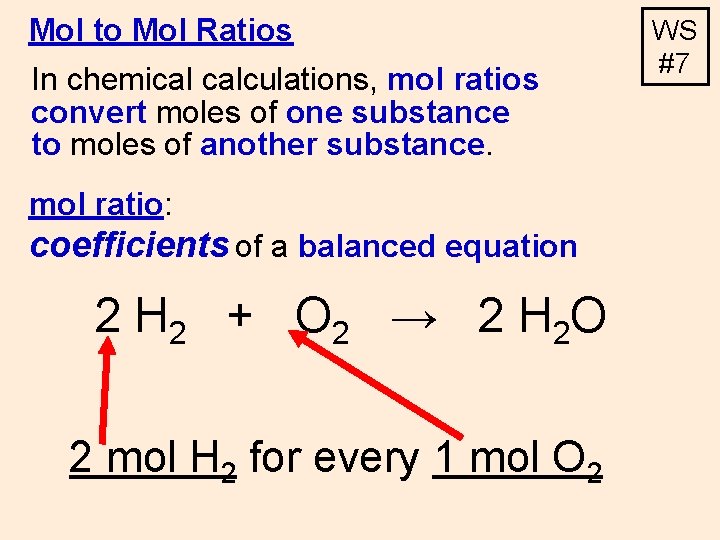
Mol to Mol Ratios In chemical calculations, mol ratios convert moles of one substance to moles of another substance. mol ratio: coefficients of a balanced equation 2 H 2 + O 2 → 2 H 2 O 2 mol H 2 for every 1 mol O 2 WS #7

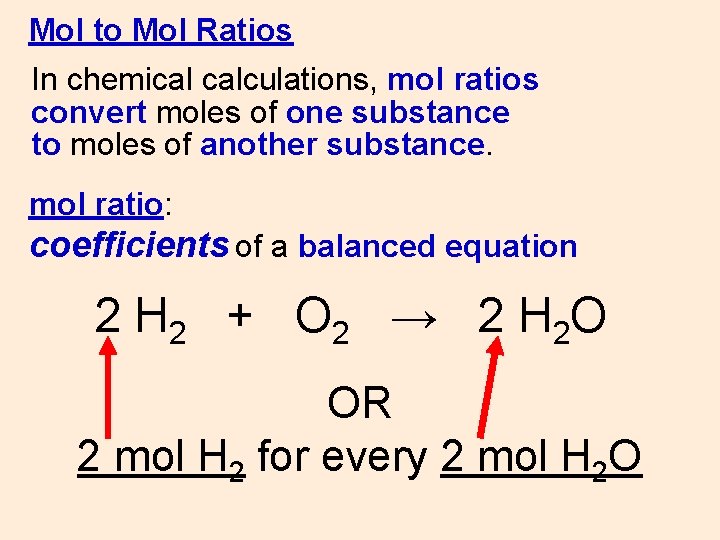
Mol to Mol Ratios In chemical calculations, mol ratios convert moles of one substance to moles of another substance. mol ratio: coefficients of a balanced equation 2 H 2 + O 2 → 2 H 2 O OR 2 mol H 2 for every 2 mol H 2 O

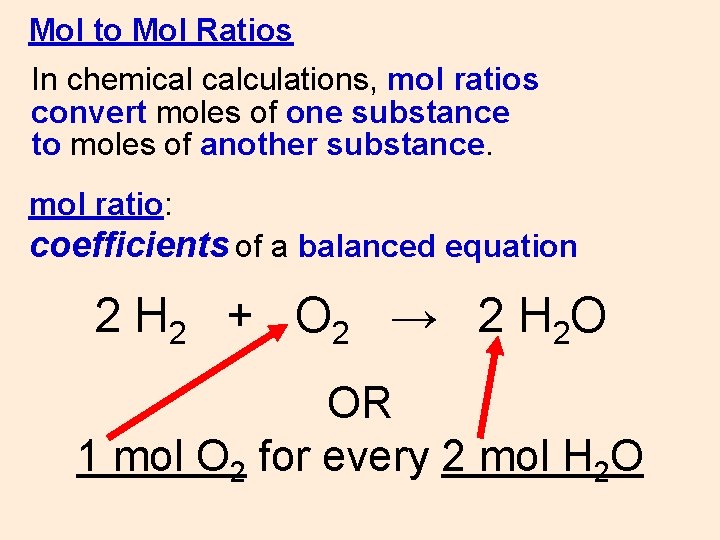
Mol to Mol Ratios In chemical calculations, mol ratios convert moles of one substance to moles of another substance. mol ratio: coefficients of a balanced equation 2 H 2 + O 2 → 2 H 2 O OR 1 mol O 2 for every 2 mol H 2 O

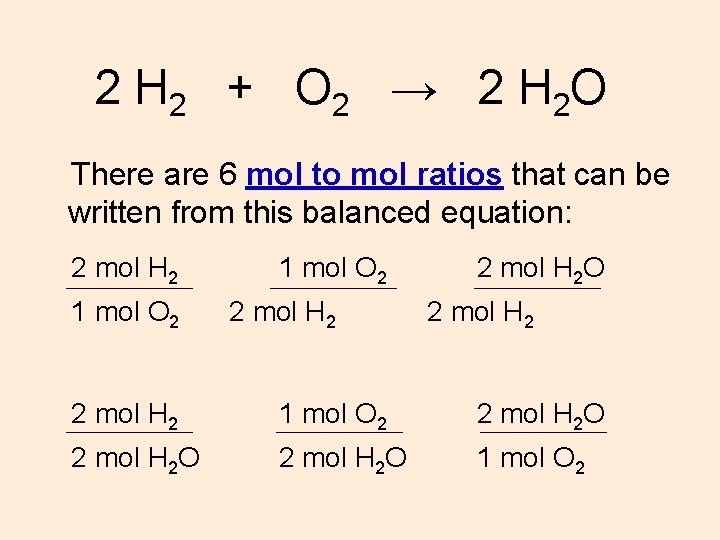
2 H 2 + O 2 → 2 H 2 O There are 6 mol to mol ratios that can be written from this balanced equation: 2 mol H 2 1 mol O 2 2 mol H 2 O 2 mol H 2 O 1 mol O 2

We must use MOLES to calculate, but… We don’t measure moles in the lab, we measure mass (in grams), but… We can’t convert grams A to grams B, so… We convert grams to moles using… Molar Mass (g/mol) from… the PT (your best friend!!!) WS #8

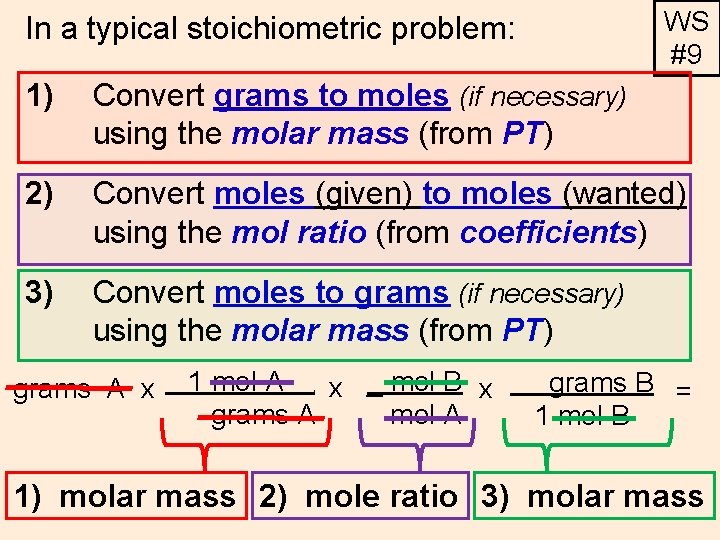
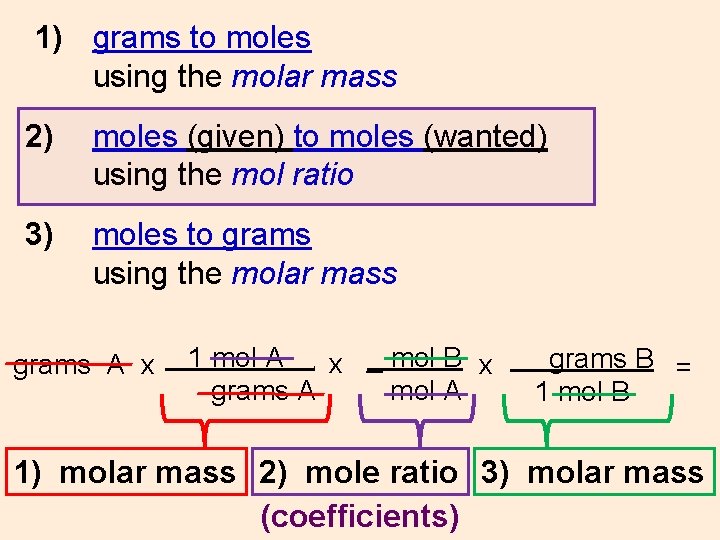
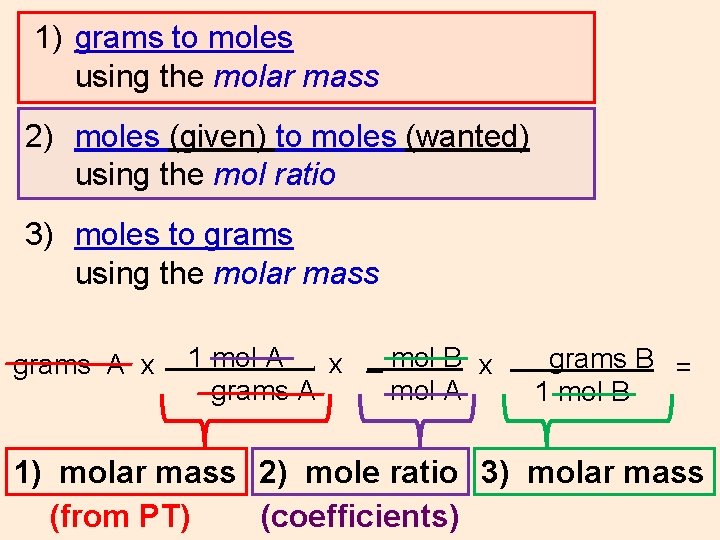
WS #9 In a typical stoichiometric problem: 1) Convert grams to moles (if necessary) using the molar mass (from PT) 2) Convert moles (given) to moles (wanted) using the mol ratio (from coefficients) 3) Convert moles to grams (if necessary) using the molar mass (from PT) grams A x 1 mol A x _ mol B x grams A mol A. grams B = 1 mol B 1) molar mass 2) mole ratio 3) molar mass

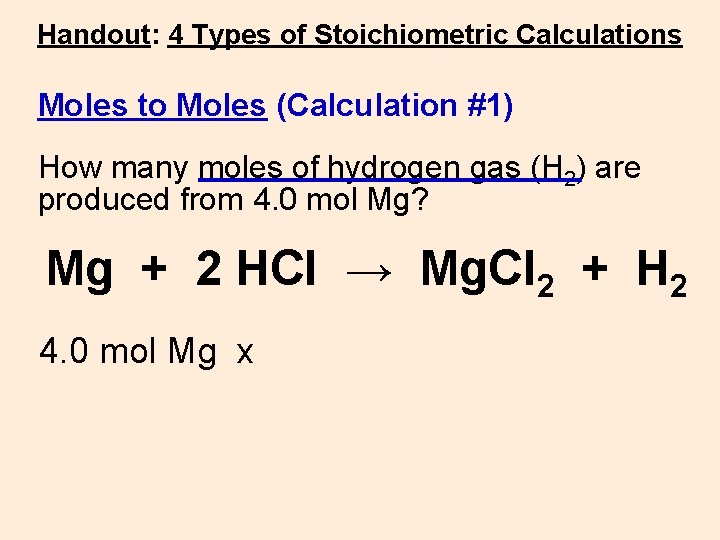
Handout: 4 Types of Stoichiometric Calculations Moles to Moles (Calculation #1) How many moles of hydrogen gas (H 2) are produced from 4. 0 mol Mg? Mg + 2 HCl → Mg. Cl 2 + H 2 4. 0 mol Mg x

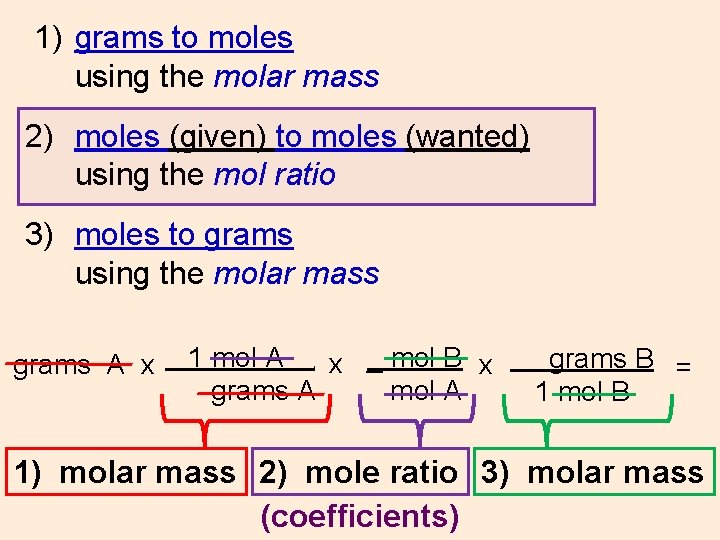
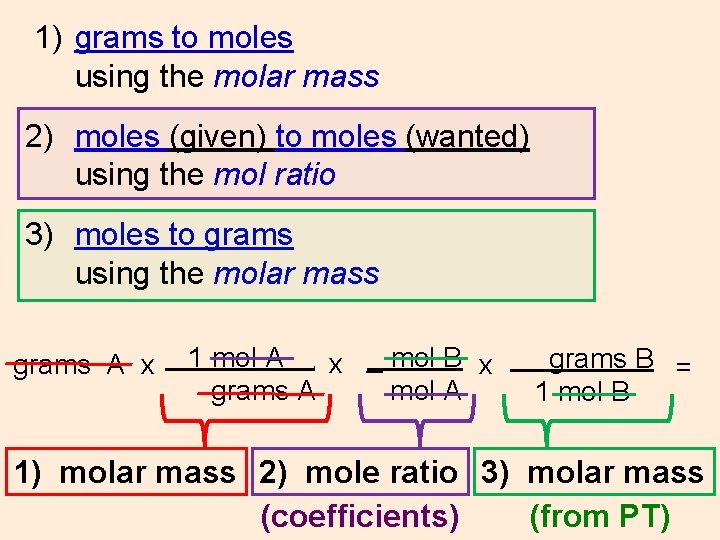
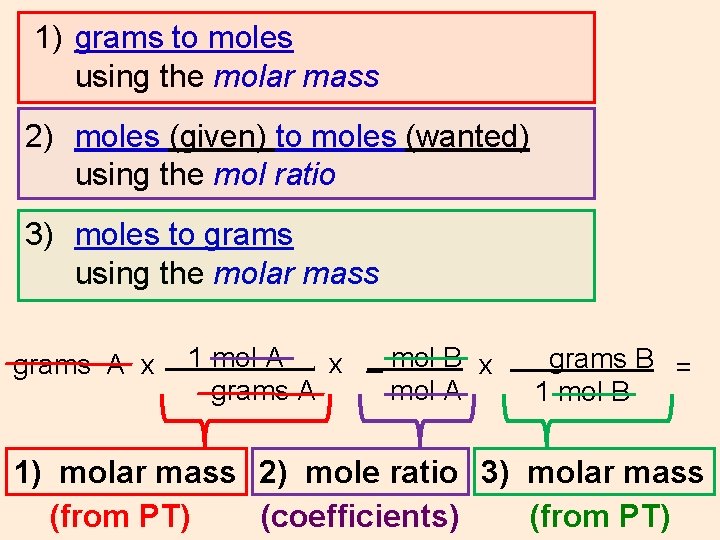
1) grams to moles using the molar mass 2) moles (given) to moles (wanted) using the mol ratio 3) moles to grams using the molar mass grams A x 1 mol A x _ mol B x grams A mol A. grams B = 1 mol B 1) molar mass 2) mole ratio 3) molar mass (coefficients)

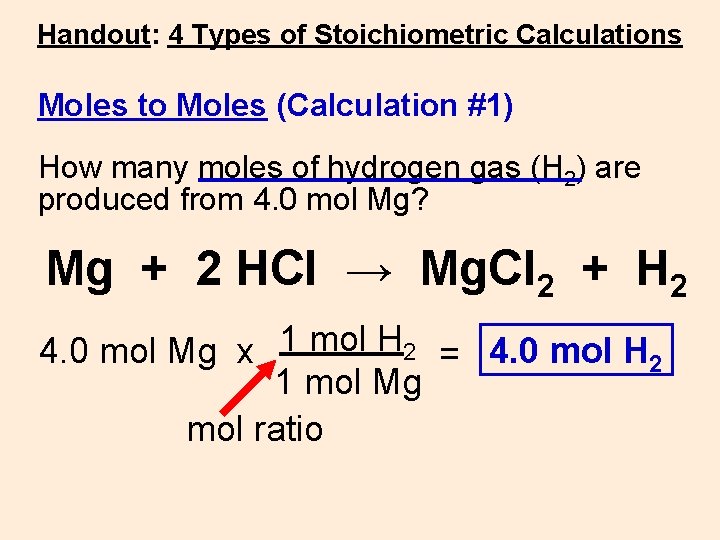
Handout: 4 Types of Stoichiometric Calculations Moles to Moles (Calculation #1) How many moles of hydrogen gas (H 2) are produced from 4. 0 mol Mg? Mg + 2 HCl → Mg. Cl 2 + H 2 4. 0 mol Mg x 1 mol H 2 = 4. 0 mol H 2 1 mol Mg mol ratio

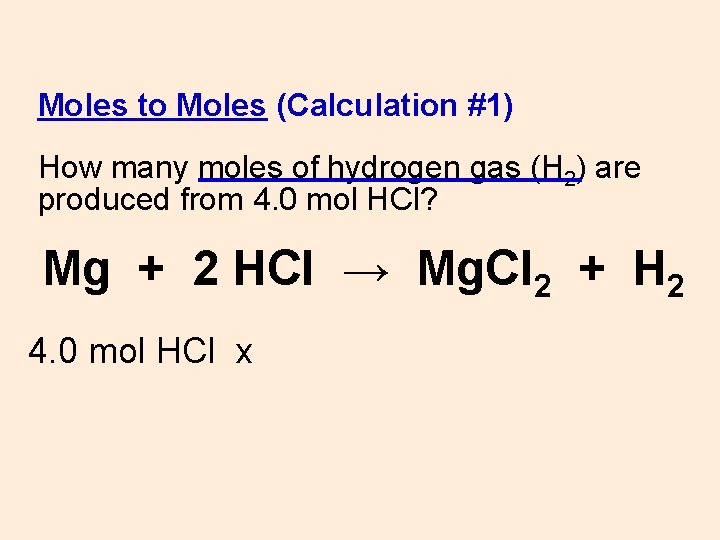
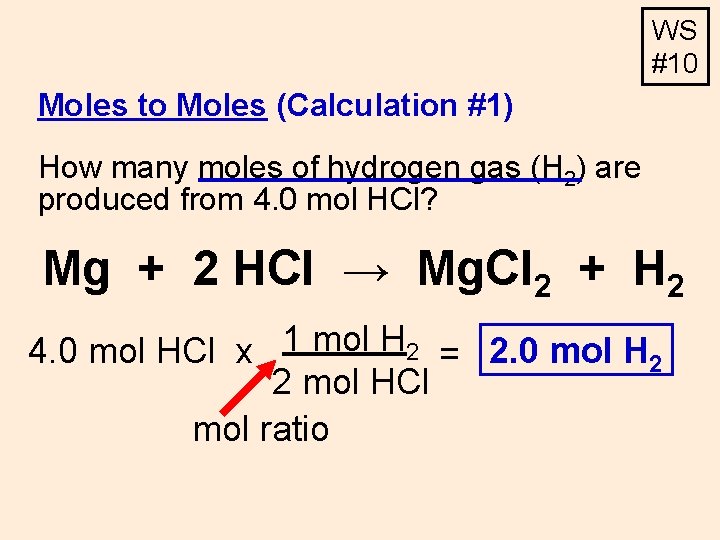
Moles to Moles (Calculation #1) How many moles of hydrogen gas (H 2) are produced from 4. 0 mol HCl? Mg + 2 HCl → Mg. Cl 2 + H 2 4. 0 mol HCl x

1) grams to moles using the molar mass 2) moles (given) to moles (wanted) using the mol ratio 3) moles to grams using the molar mass grams A x 1 mol A x _ mol B x grams A mol A. grams B = 1 mol B 1) molar mass 2) mole ratio 3) molar mass (coefficients)

WS #10 Moles to Moles (Calculation #1) How many moles of hydrogen gas (H 2) are produced from 4. 0 mol HCl? Mg + 2 HCl → Mg. Cl 2 + H 2 4. 0 mol HCl x 1 mol H 2 = 2. 0 mol H 2 2 mol HCl mol ratio

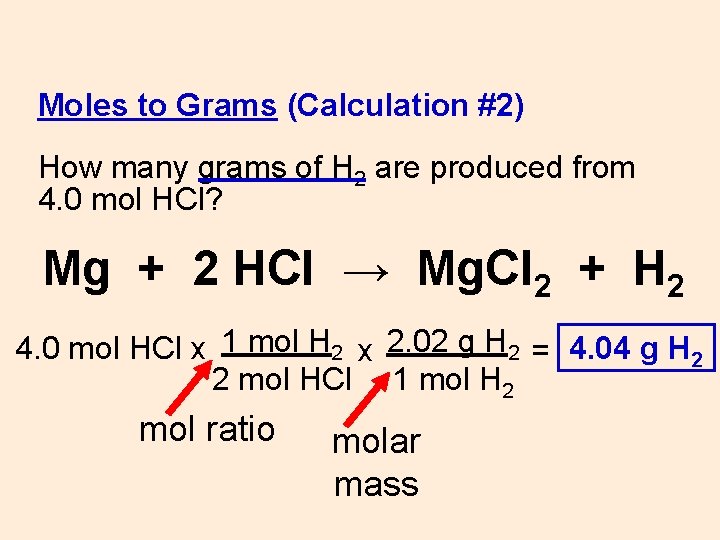
Moles to Grams (Calculation #2) How many grams of H 2 are produced from 4. 0 mol HCl? Mg + 2 HCl → Mg. Cl 2 + H 2 4. 0 mol HCl x

1) grams to moles using the molar mass 2) moles (given) to moles (wanted) using the mol ratio 3) moles to grams using the molar mass grams A x 1 mol A x _ mol B x grams A mol A. grams B = 1 mol B 1) molar mass 2) mole ratio 3) molar mass (coefficients) (from PT)

Moles to Grams (Calculation #2) How many grams of H 2 are produced from 4. 0 mol HCl? Mg + 2 HCl → Mg. Cl 2 + H 2 4. 0 mol HCl x 1 mol H 2 x 2. 02 g H 2 = 4. 04 g H 2 2 mol HCl 1 mol H 2 mol ratio molar mass

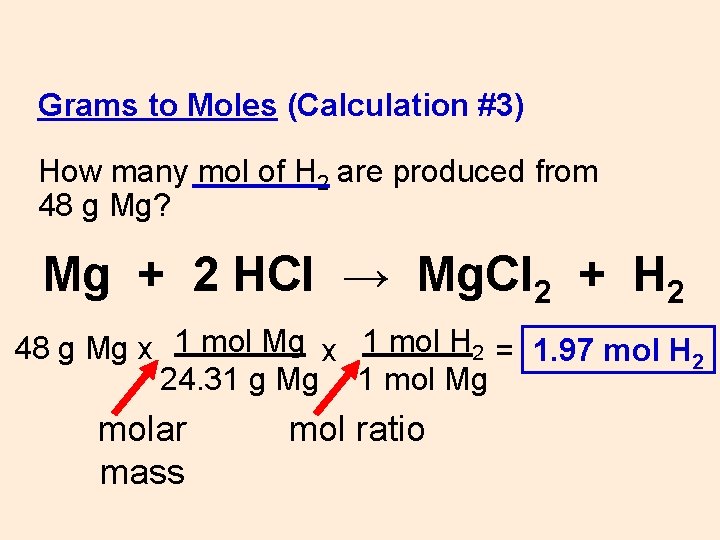
Grams to Moles (Calculation #3) How many mol of H 2 are produced from 48 g Mg? Mg + 2 HCl → Mg. Cl 2 + H 2 48 g Mg x

1) grams to moles using the molar mass 2) moles (given) to moles (wanted) using the mol ratio 3) moles to grams using the molar mass grams A x 1 mol A x _ mol B x grams A mol A. grams B = 1 mol B 1) molar mass 2) mole ratio 3) molar mass (from PT) (coefficients)

Grams to Moles (Calculation #3) How many mol of H 2 are produced from 48 g Mg? Mg + 2 HCl → Mg. Cl 2 + H 2 48 g Mg x 1 mol H 2 = 1. 97 mol H 2 24. 31 g Mg 1 mol Mg molar mass mol ratio

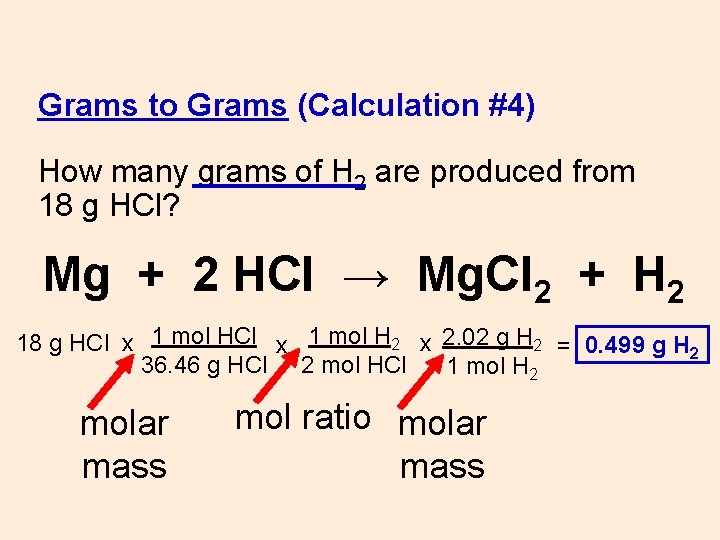
Grams to Grams (Calculation #4) How many grams of H 2 are produced from 18 g HCl? Mg + 2 HCl → Mg. Cl 2 + H 2 18 g HCl x

1) grams to moles using the molar mass 2) moles (given) to moles (wanted) using the mol ratio 3) moles to grams using the molar mass grams A x 1 mol A x _ mol B x grams A mol A. grams B = 1 mol B 1) molar mass 2) mole ratio 3) molar mass (from PT) (coefficients) (from PT)

Grams to Grams (Calculation #4) How many grams of H 2 are produced from 18 g HCl? Mg + 2 HCl → Mg. Cl 2 + H 2 18 g HCl x 1 mol H 2 x 2. 02 g H 2 = 0. 499 g H 2 36. 46 g HCl 2 mol HCl 1 mol H 2 molar mass mol ratio molar mass

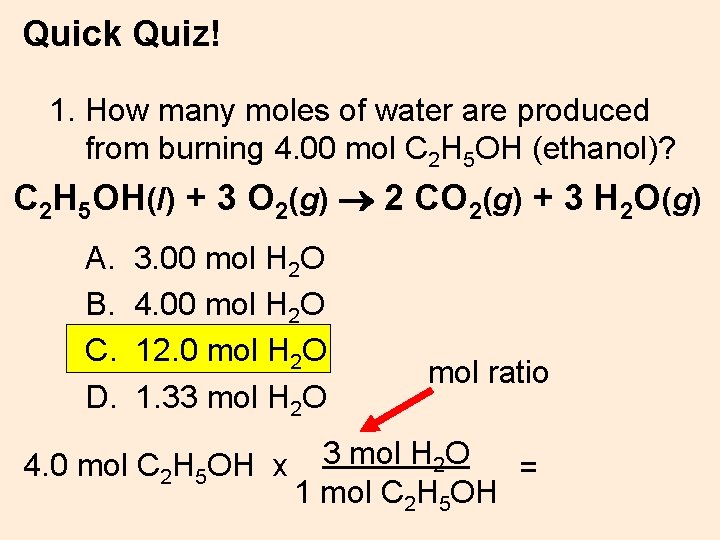
Quick Quiz! 1. How many moles of water are produced from burning 4. 00 mol C 2 H 5 OH (ethanol)? C 2 H 5 OH(l) + 3 O 2(g) 2 CO 2(g) + 3 H 2 O(g) A. B. C. D. 3. 00 mol H 2 O 4. 00 mol H 2 O 12. 0 mol H 2 O 1. 33 mol H 2 O 4. 0 mol C 2 H 5 OH x mol ratio 3 mol H 2 O = 1 mol C 2 H 5 OH

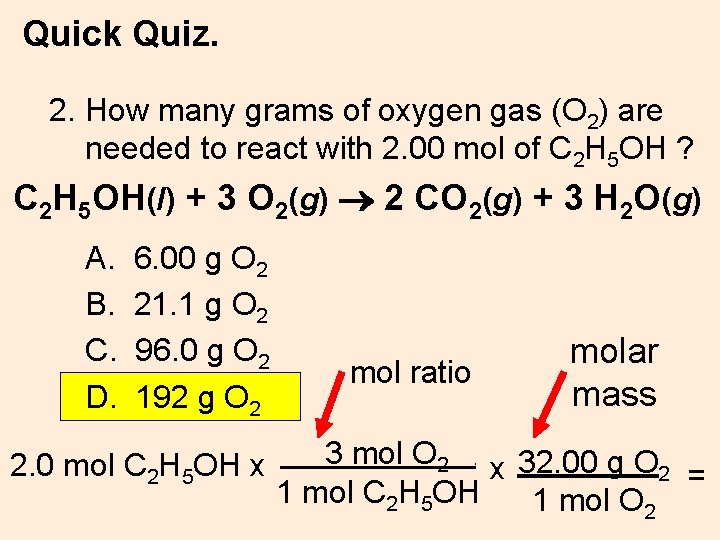
Quick Quiz. 2. How many grams of oxygen gas (O 2) are needed to react with 2. 00 mol of C 2 H 5 OH ? C 2 H 5 OH(l) + 3 O 2(g) 2 CO 2(g) + 3 H 2 O(g) A. B. C. D. 6. 00 g O 2 21. 1 g O 2 96. 0 g O 2 192 g O 2 2. 0 mol C 2 H 5 OH x molar mass mol ratio 3 mol O 2 x 32. 00 g O 2 = 1 mol C 2 H 5 OH 1 mol O 2.