Chapter 10 Answers MoleParticle Conversions 1 How many



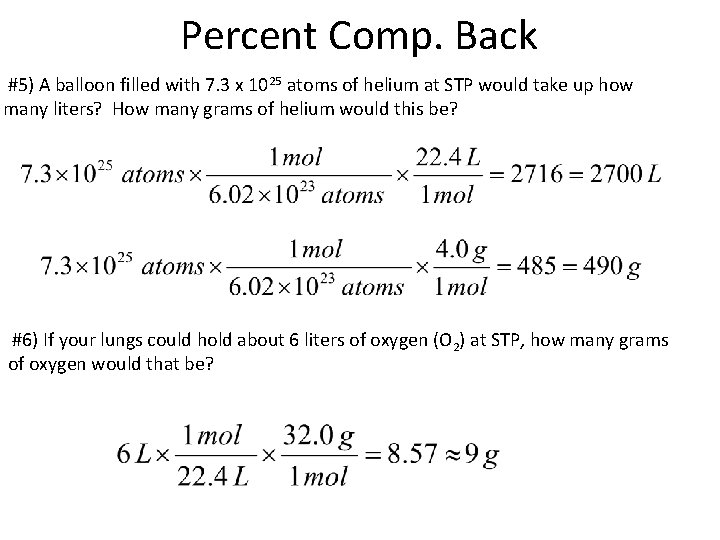


- Slides: 53

Chapter 10 Answers

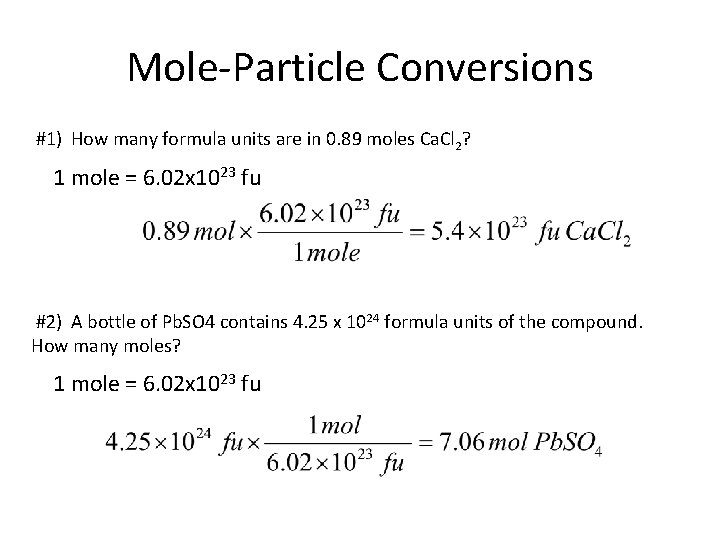
Mole-Particle Conversions #1) How many formula units are in 0. 89 moles Ca. Cl 2? 1 mole = 6. 02 x 1023 fu #2) A bottle of Pb. SO 4 contains 4. 25 x 1024 formula units of the compound. How many moles? 1 mole = 6. 02 x 1023 fu

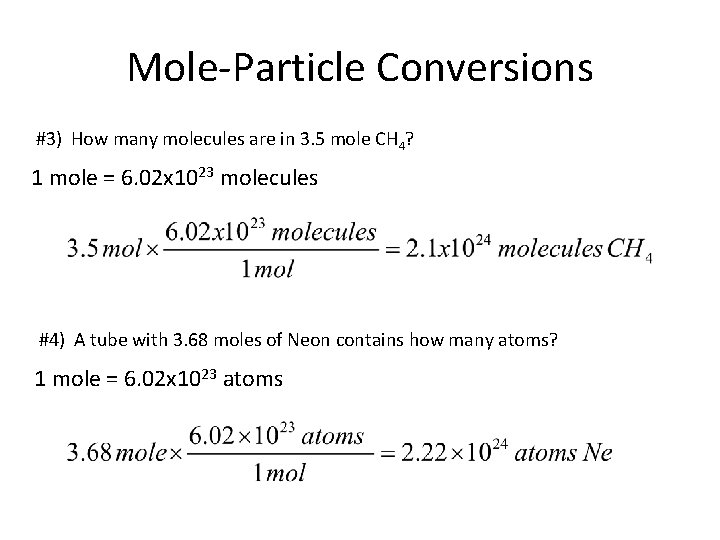
Mole-Particle Conversions #3) How many molecules are in 3. 5 mole CH 4? 1 mole = 6. 02 x 1023 molecules #4) A tube with 3. 68 moles of Neon contains how many atoms? 1 mole = 6. 02 x 1023 atoms

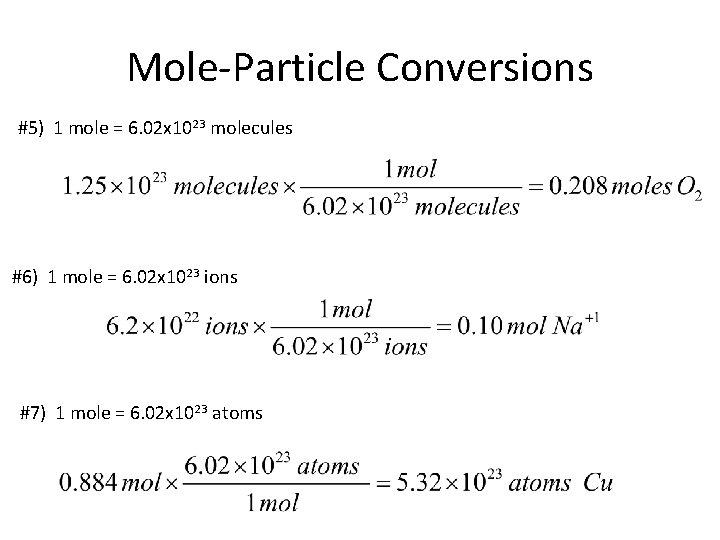
Mole-Particle Conversions #5) 1 mole = 6. 02 x 1023 molecules #6) 1 mole = 6. 02 x 1023 ions #7) 1 mole = 6. 02 x 1023 atoms

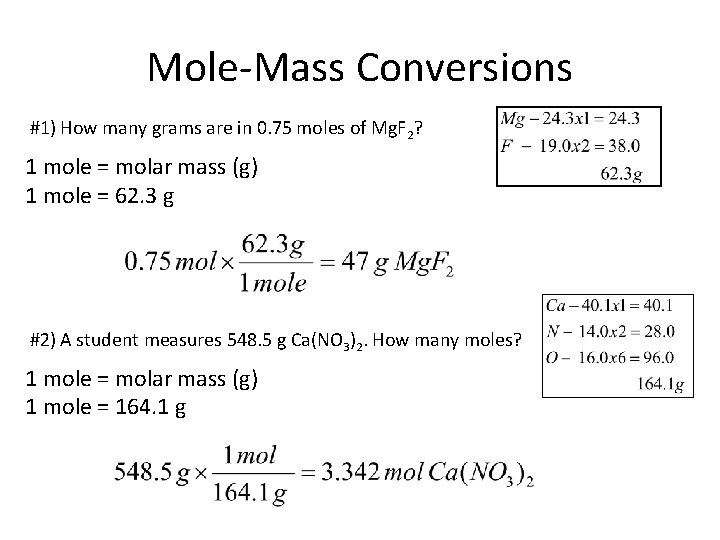
Mole-Mass Conversions #1) How many grams are in 0. 75 moles of Mg. F 2? 1 mole = molar mass (g) 1 mole = 62. 3 g #2) A student measures 548. 5 g Ca(NO 3)2. How many moles? 1 mole = molar mass (g) 1 mole = 164. 1 g

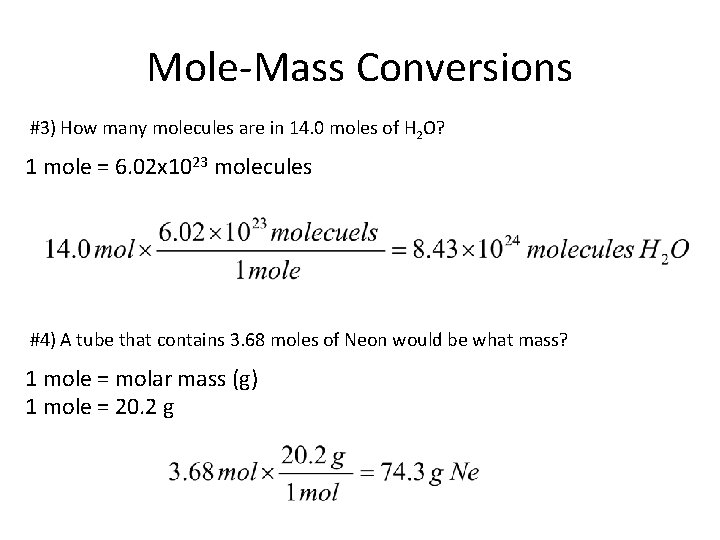
Mole-Mass Conversions #3) How many molecules are in 14. 0 moles of H 2 O? 1 mole = 6. 02 x 1023 molecules #4) A tube that contains 3. 68 moles of Neon would be what mass? 1 mole = molar mass (g) 1 mole = 20. 2 g

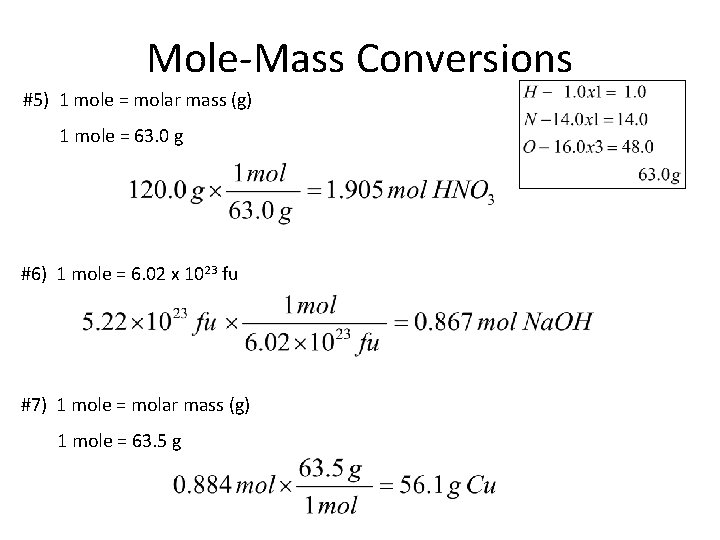
Mole-Mass Conversions #5) 1 mole = molar mass (g) 1 mole = 63. 0 g #6) 1 mole = 6. 02 x 1023 fu #7) 1 mole = molar mass (g) 1 mole = 63. 5 g

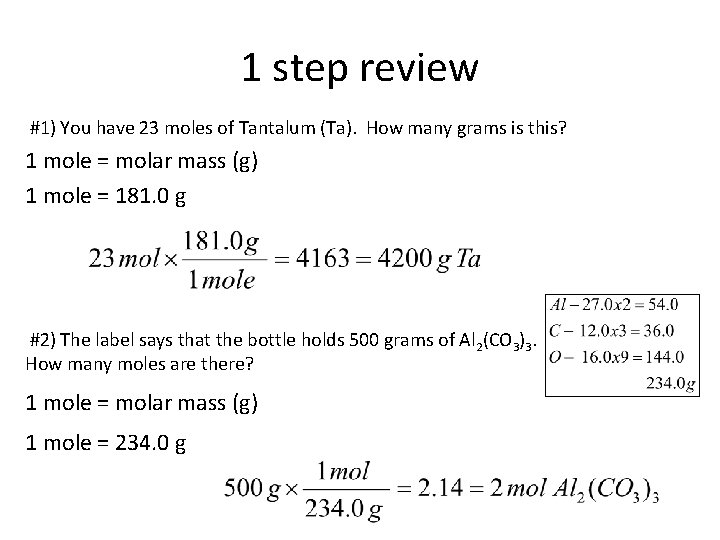
1 step review #1) You have 23 moles of Tantalum (Ta). How many grams is this? 1 mole = molar mass (g) 1 mole = 181. 0 g #2) The label says that the bottle holds 500 grams of Al 2(CO 3)3. How many moles are there? 1 mole = molar mass (g) 1 mole = 234. 0 g

1 step review #3) How many moles of argon atoms are present in 11. 2 L of argon gas at STP? 1 mole = 22. 4 L #4) Your silver watchband masses out at 326 g. How many moles do you have? 1 mole = molar mass (g) 1 mole = 107. 9 g

1 step review #5) How many molecules are in 0. 400 moles of N 2 O 5? 1 mole = 6. 02 x 1023 molecules #6) Determine the volume, in liters, occupied by 0. 030 moles of a gas at STP. 1 mole = 22. 4 L

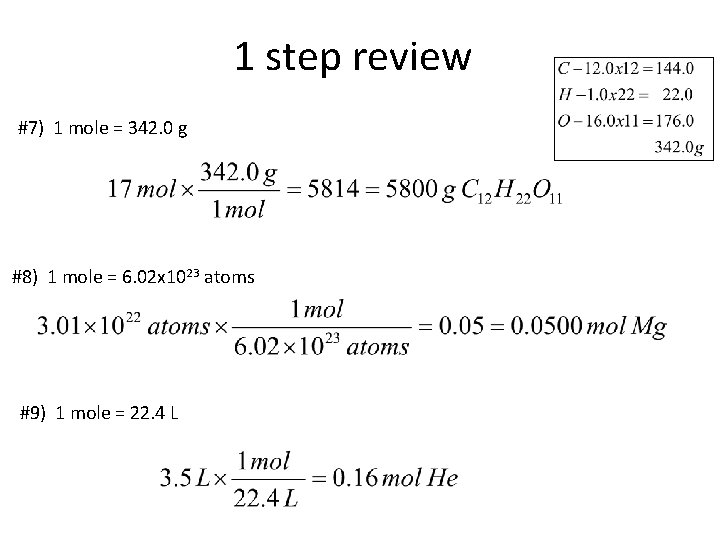
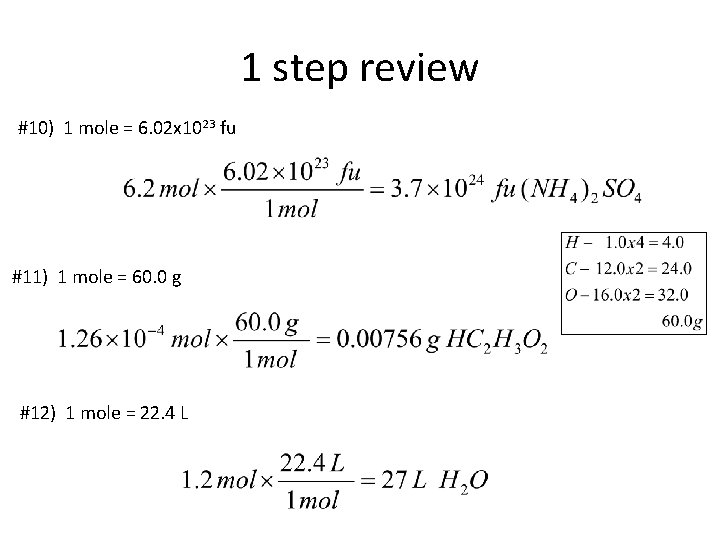
1 step review #7) 1 mole = 342. 0 g #8) 1 mole = 6. 02 x 1023 atoms #9) 1 mole = 22. 4 L

1 step review #10) 1 mole = 6. 02 x 1023 fu #11) 1 mole = 60. 0 g #12) 1 mole = 22. 4 L

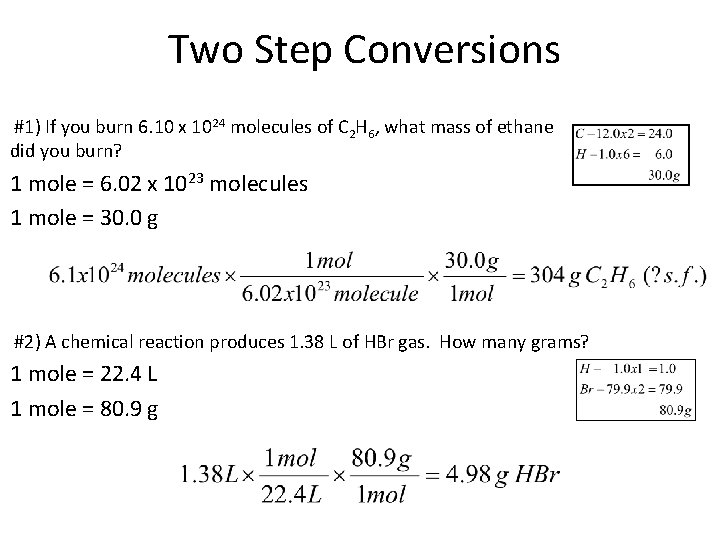
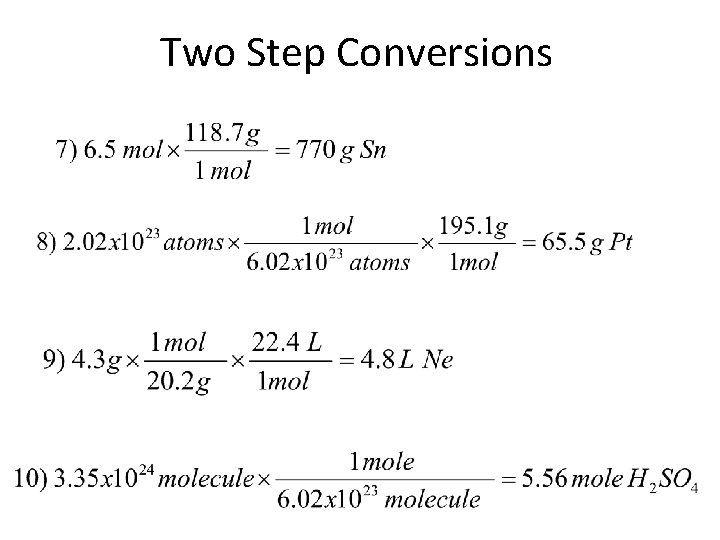
Two Step Conversions #1) If you burn 6. 10 x 1024 molecules of C 2 H 6, what mass of ethane did you burn? 1 mole = 6. 02 x 1023 molecules 1 mole = 30. 0 g #2) A chemical reaction produces 1. 38 L of HBr gas. How many grams? 1 mole = 22. 4 L 1 mole = 80. 9 g

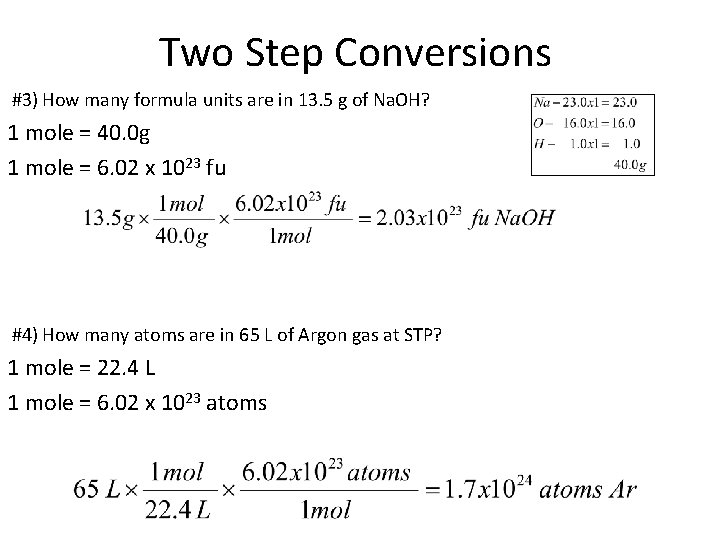
Two Step Conversions #3) How many formula units are in 13. 5 g of Na. OH? 1 mole = 40. 0 g 1 mole = 6. 02 x 1023 fu #4) How many atoms are in 65 L of Argon gas at STP? 1 mole = 22. 4 L 1 mole = 6. 02 x 1023 atoms

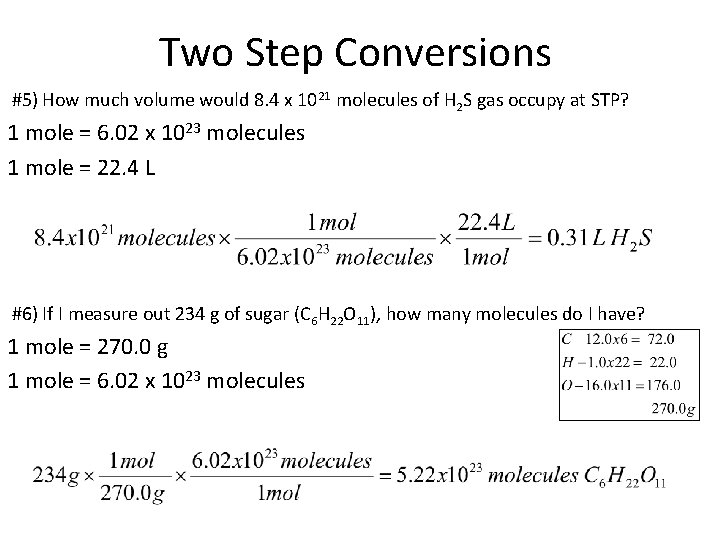
Two Step Conversions #5) How much volume would 8. 4 x 1021 molecules of H 2 S gas occupy at STP? 1 mole = 6. 02 x 1023 molecules 1 mole = 22. 4 L #6) If I measure out 234 g of sugar (C 6 H 22 O 11), how many molecules do I have? 1 mole = 270. 0 g 1 mole = 6. 02 x 1023 molecules

Two Step Conversions

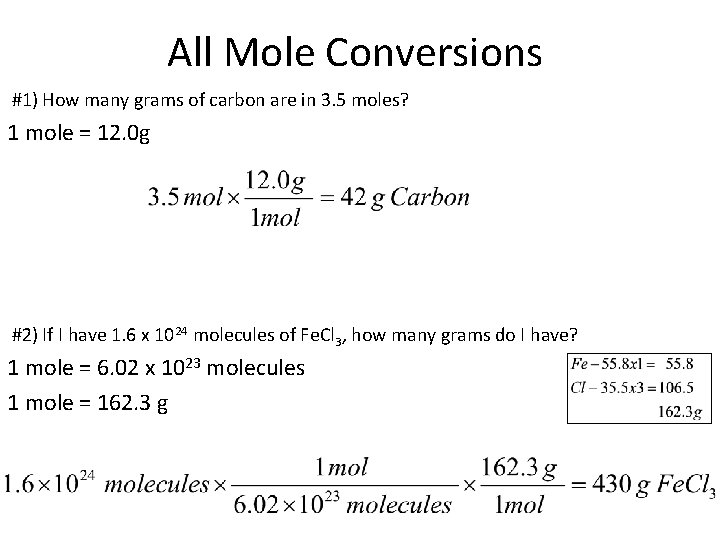
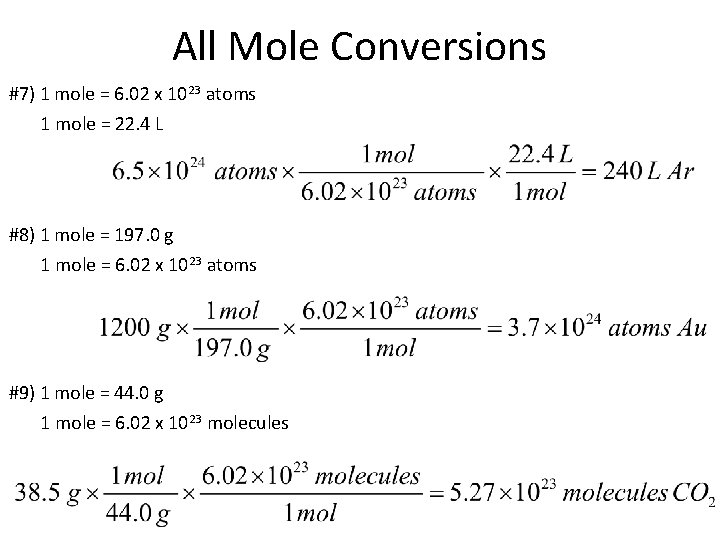
All Mole Conversions #1) How many grams of carbon are in 3. 5 moles? 1 mole = 12. 0 g #2) If I have 1. 6 x 1024 molecules of Fe. Cl 3, how many grams do I have? 1 mole = 6. 02 x 1023 molecules 1 mole = 162. 3 g

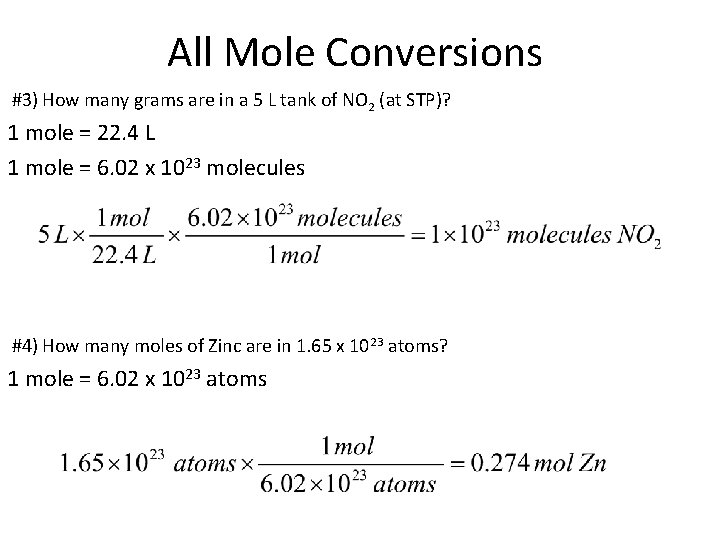
All Mole Conversions #3) How many grams are in a 5 L tank of NO 2 (at STP)? 1 mole = 22. 4 L 1 mole = 6. 02 x 1023 molecules #4) How many moles of Zinc are in 1. 65 x 1023 atoms? 1 mole = 6. 02 x 1023 atoms

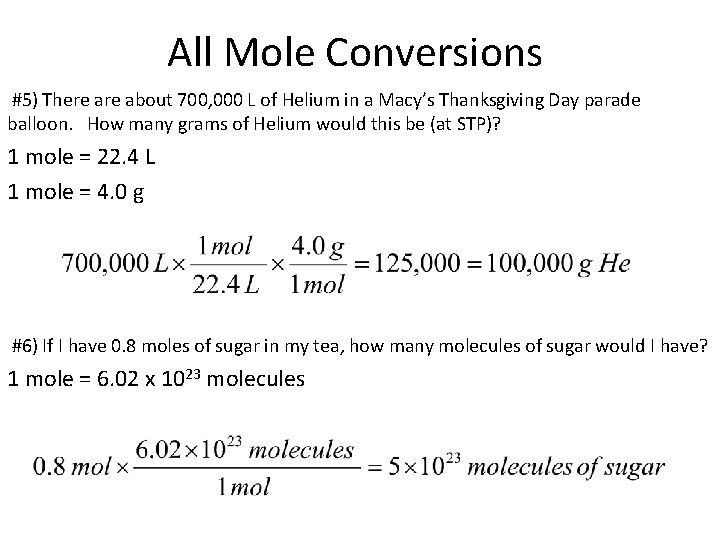
All Mole Conversions #5) There about 700, 000 L of Helium in a Macy’s Thanksgiving Day parade balloon. How many grams of Helium would this be (at STP)? 1 mole = 22. 4 L 1 mole = 4. 0 g #6) If I have 0. 8 moles of sugar in my tea, how many molecules of sugar would I have? 1 mole = 6. 02 x 1023 molecules

All Mole Conversions #7) 1 mole = 6. 02 x 1023 atoms 1 mole = 22. 4 L #8) 1 mole = 197. 0 g 1 mole = 6. 02 x 1023 atoms #9) 1 mole = 44. 0 g 1 mole = 6. 02 x 1023 molecules

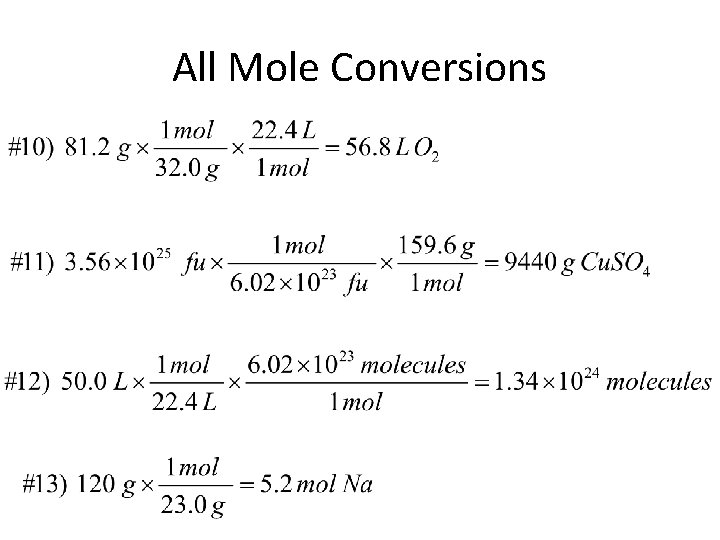
All Mole Conversions

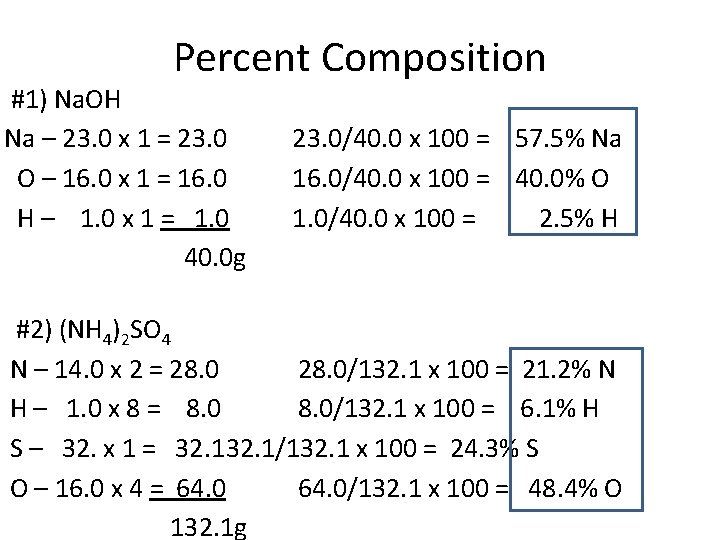
Percent Composition #1) Na. OH Na – 23. 0 x 1 = 23. 0 O – 16. 0 x 1 = 16. 0 H – 1. 0 x 1 = 1. 0 40. 0 g 23. 0/40. 0 x 100 = 57. 5% Na 16. 0/40. 0 x 100 = 40. 0% O 1. 0/40. 0 x 100 = 2. 5% H #2) (NH 4)2 SO 4 N – 14. 0 x 2 = 28. 0/132. 1 x 100 = 21. 2% N H – 1. 0 x 8 = 8. 0/132. 1 x 100 = 6. 1% H S – 32. x 1 = 32. 1/132. 1 x 100 = 24. 3% S O – 16. 0 x 4 = 64. 0/132. 1 x 100 = 48. 4% O 132. 1 g

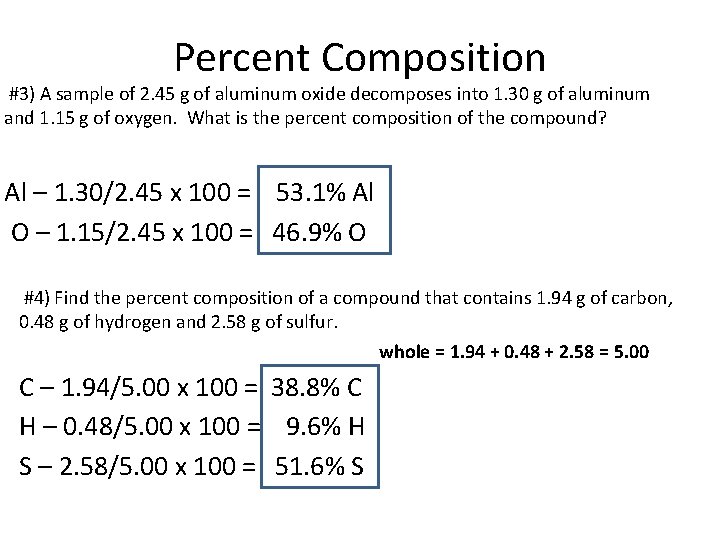
Percent Composition #3) A sample of 2. 45 g of aluminum oxide decomposes into 1. 30 g of aluminum and 1. 15 g of oxygen. What is the percent composition of the compound? Al – 1. 30/2. 45 x 100 = 53. 1% Al O – 1. 15/2. 45 x 100 = 46. 9% O #4) Find the percent composition of a compound that contains 1. 94 g of carbon, 0. 48 g of hydrogen and 2. 58 g of sulfur. whole = 1. 94 + 0. 48 + 2. 58 = 5. 00 C – 1. 94/5. 00 x 100 = 38. 8% C H – 0. 48/5. 00 x 100 = 9. 6% H S – 2. 58/5. 00 x 100 = 51. 6% S

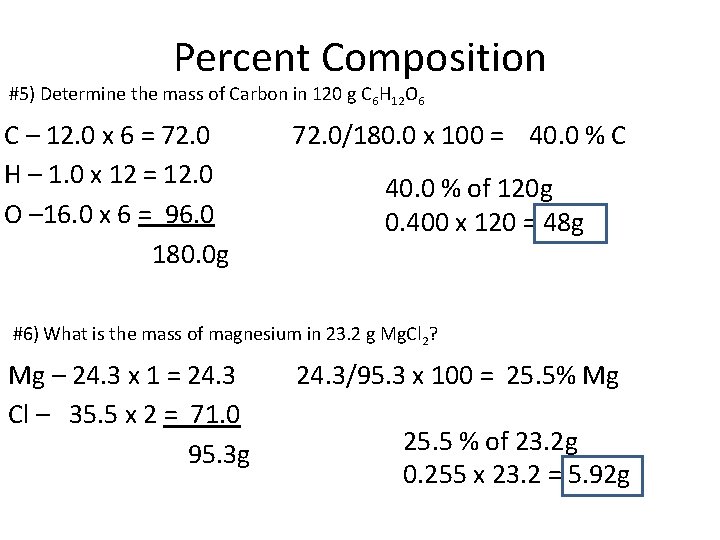
Percent Composition #5) Determine the mass of Carbon in 120 g C 6 H 12 O 6 C – 12. 0 x 6 = 72. 0 H – 1. 0 x 12 = 12. 0 O – 16. 0 x 6 = 96. 0 180. 0 g 72. 0/180. 0 x 100 = 40. 0 % C 40. 0 % of 120 g 0. 400 x 120 = 48 g #6) What is the mass of magnesium in 23. 2 g Mg. Cl 2? Mg – 24. 3 x 1 = 24. 3 Cl – 35. 5 x 2 = 71. 0 95. 3 g 24. 3/95. 3 x 100 = 25. 5% Mg 25. 5 % of 23. 2 g 0. 255 x 23. 2 = 5. 92 g

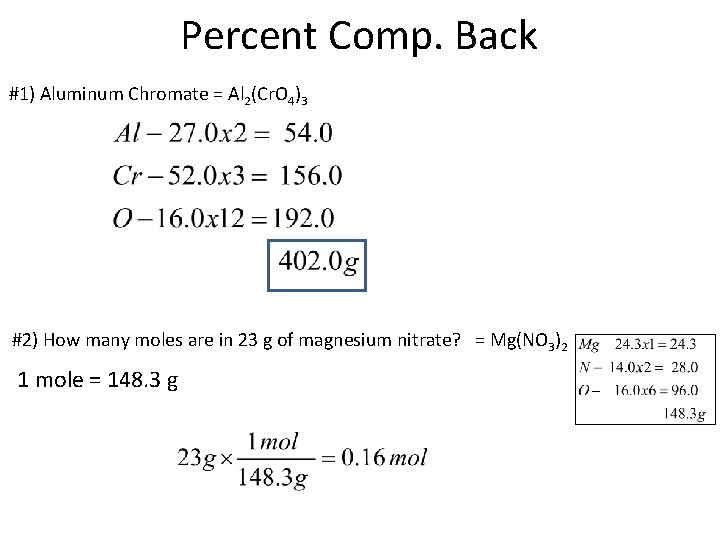
Percent Comp. Back #1) Aluminum Chromate = Al 2(Cr. O 4)3 #2) How many moles are in 23 g of magnesium nitrate? = Mg(NO 3)2 1 mole = 148. 3 g

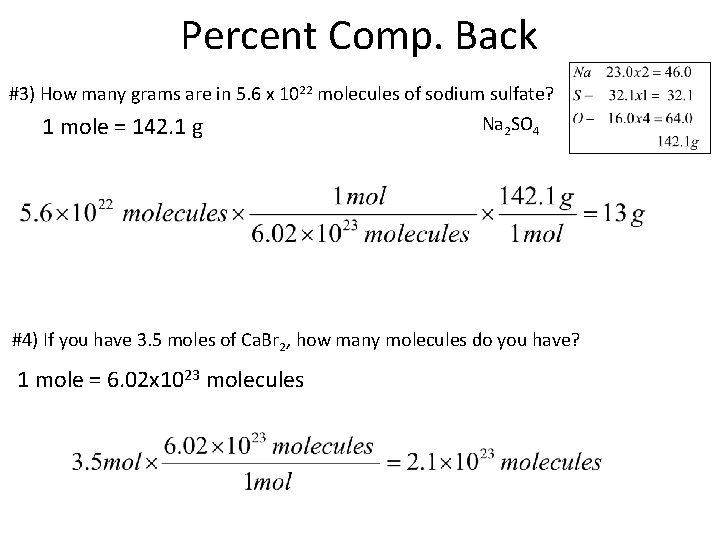
Percent Comp. Back #3) How many grams are in 5. 6 x 1022 molecules of sodium sulfate? 1 mole = 142. 1 g Na 2 SO 4 #4) If you have 3. 5 moles of Ca. Br 2, how many molecules do you have? 1 mole = 6. 02 x 1023 molecules
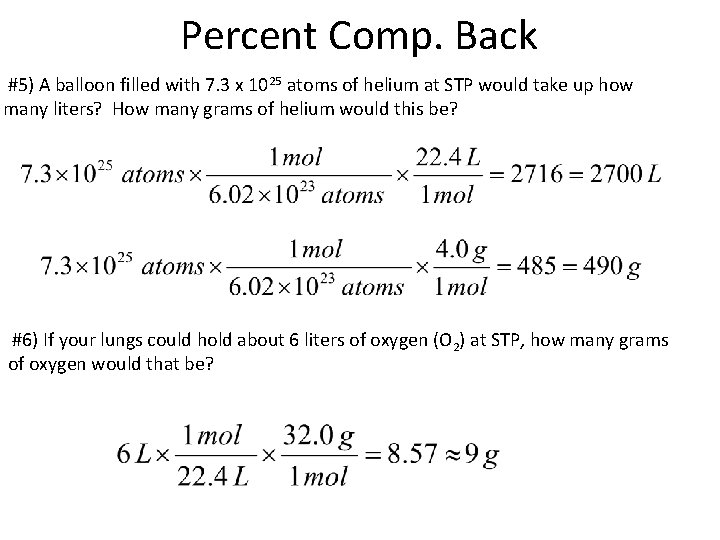
Percent Comp. Back #5) A balloon filled with 7. 3 x 1025 atoms of helium at STP would take up how many liters? How many grams of helium would this be? #6) If your lungs could hold about 6 liters of oxygen (O 2) at STP, how many grams of oxygen would that be?

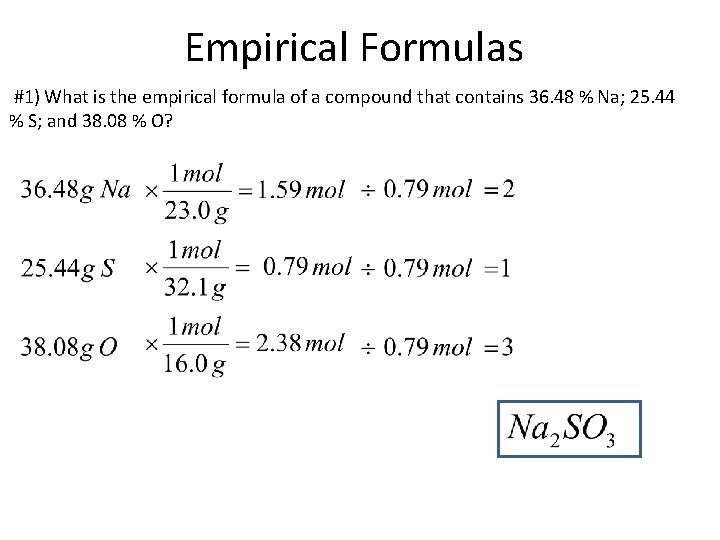
Empirical Formulas #1) What is the empirical formula of a compound that contains 36. 48 % Na; 25. 44 % S; and 38. 08 % O?

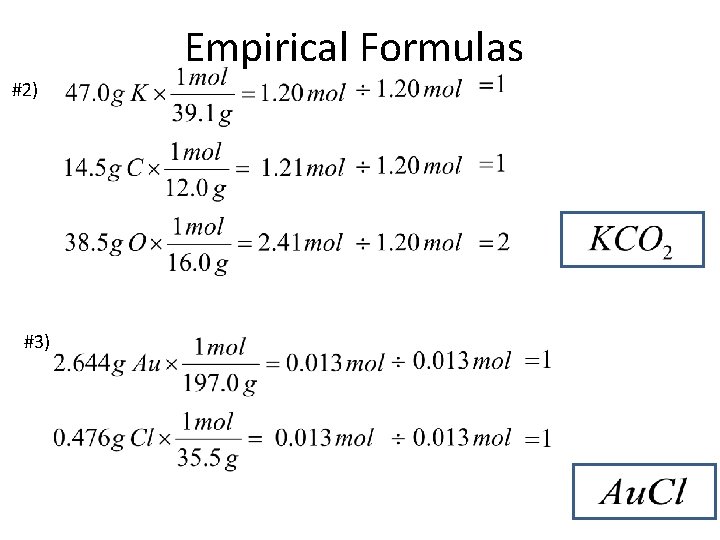
Empirical Formulas #2) #3)

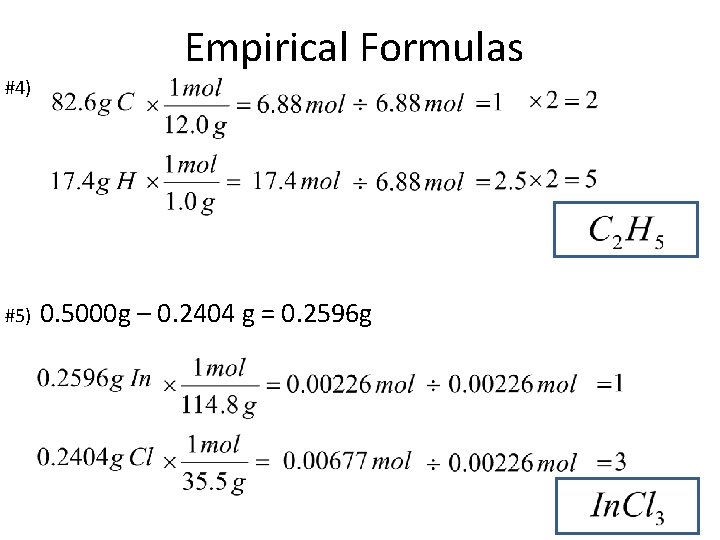
Empirical Formulas #4) #5) 0. 5000 g – 0. 2404 g = 0. 2596 g

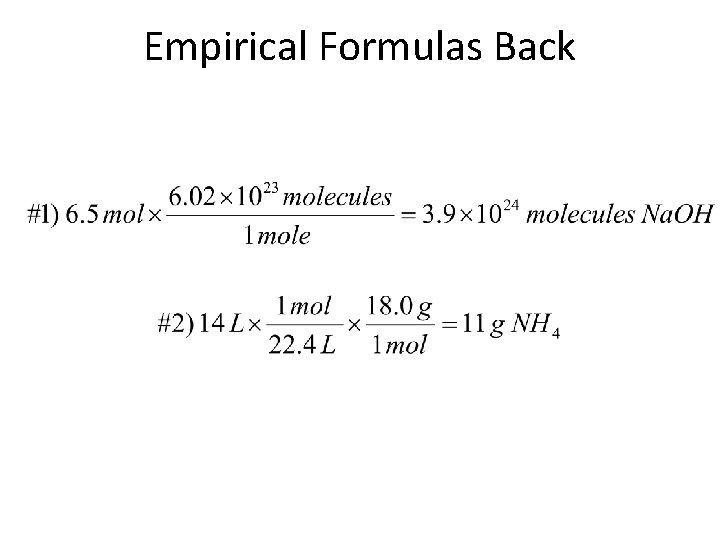
Empirical Formulas Back

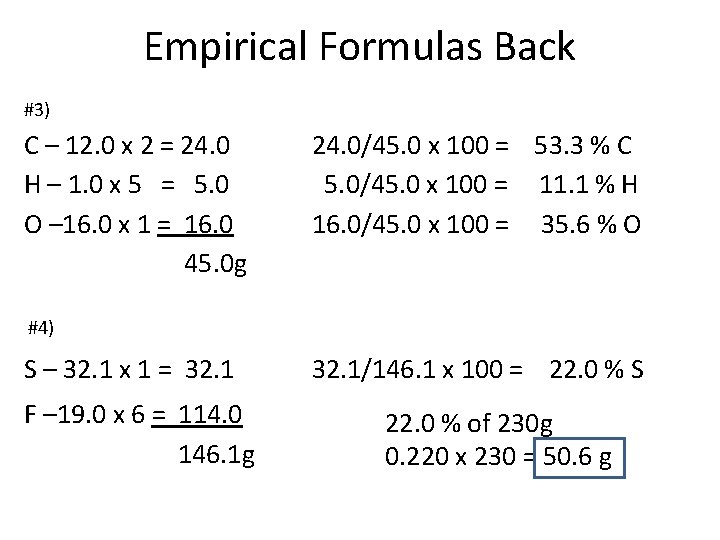
Empirical Formulas Back #3) C – 12. 0 x 2 = 24. 0 H – 1. 0 x 5 = 5. 0 O – 16. 0 x 1 = 16. 0 45. 0 g 24. 0/45. 0 x 100 = 53. 3 % C 5. 0/45. 0 x 100 = 11. 1 % H 16. 0/45. 0 x 100 = 35. 6 % O #4) S – 32. 1 x 1 = 32. 1 F – 19. 0 x 6 = 114. 0 146. 1 g 32. 1/146. 1 x 100 = 22. 0 % S 22. 0 % of 230 g 0. 220 x 230 = 50. 6 g

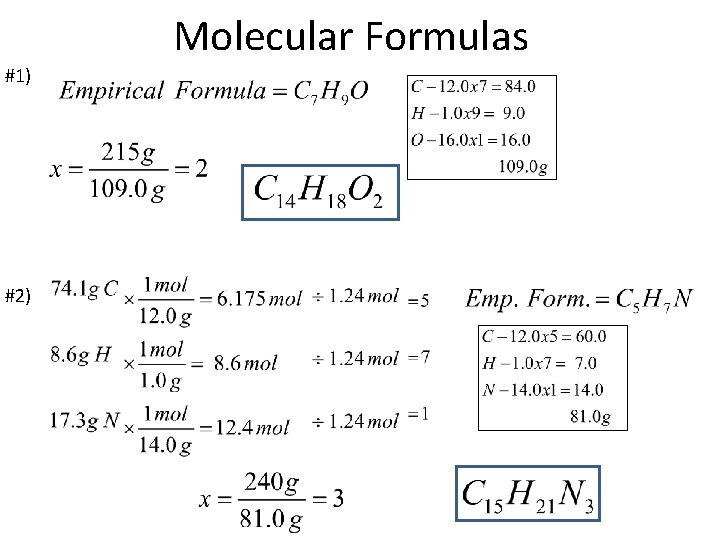
Molecular Formulas #1) #2)

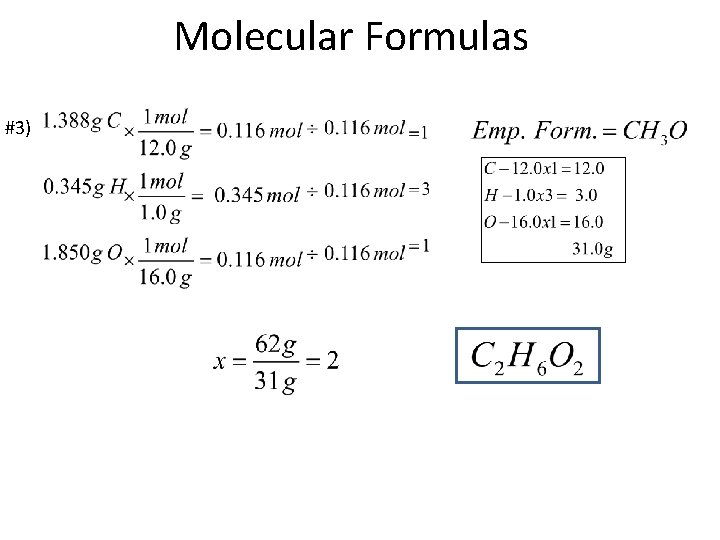
Molecular Formulas #3)

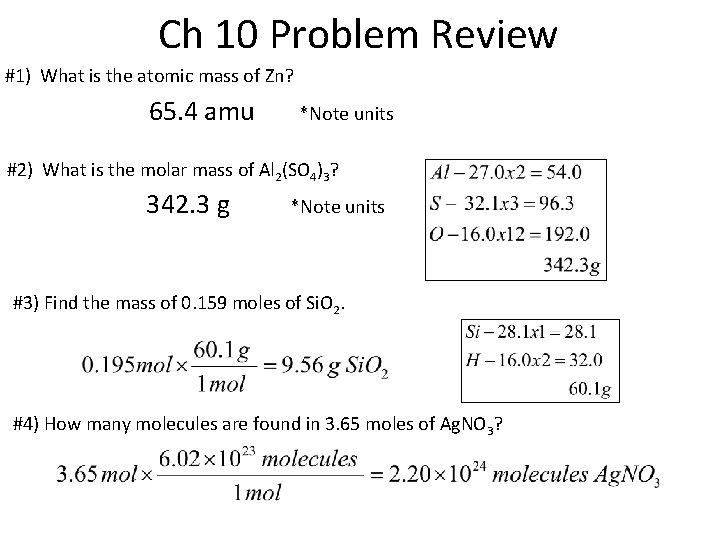
Ch 10 Problem Review #1) What is the atomic mass of Zn? 65. 4 amu *Note units #2) What is the molar mass of Al 2(SO 4)3? 342. 3 g *Note units #3) Find the mass of 0. 159 moles of Si. O 2. #4) How many molecules are found in 3. 65 moles of Ag. NO 3?

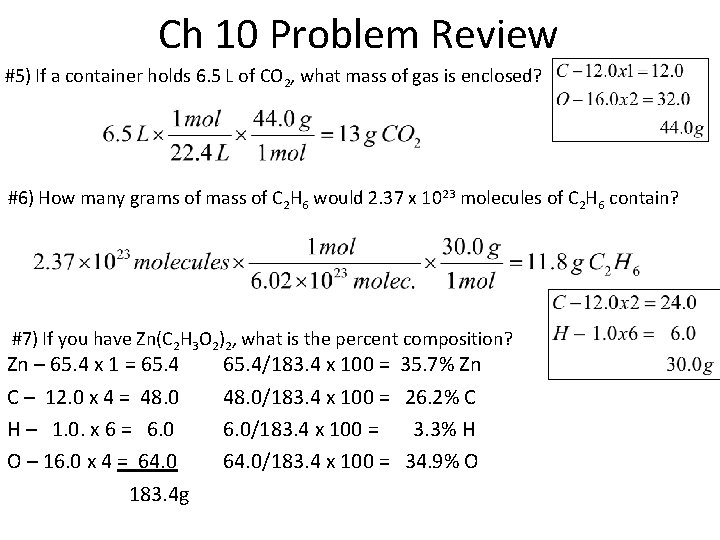
Ch 10 Problem Review #5) If a container holds 6. 5 L of CO 2, what mass of gas is enclosed? #6) How many grams of mass of C 2 H 6 would 2. 37 x 1023 molecules of C 2 H 6 contain? #7) If you have Zn(C 2 H 3 O 2)2, what is the percent composition? Zn – 65. 4 x 1 = 65. 4 C – 12. 0 x 4 = 48. 0 H – 1. 0. x 6 = 6. 0 O – 16. 0 x 4 = 64. 0 183. 4 g 65. 4/183. 4 x 100 = 35. 7% Zn 48. 0/183. 4 x 100 = 26. 2% C 6. 0/183. 4 x 100 = 3. 3% H 64. 0/183. 4 x 100 = 34. 9% O

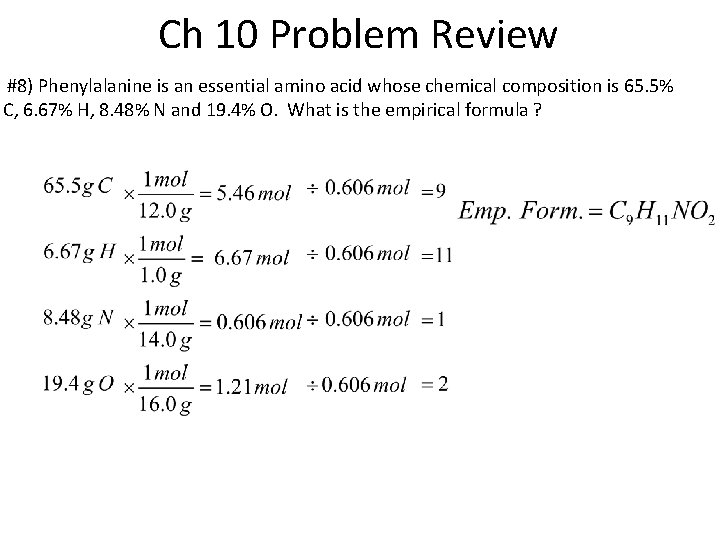
Ch 10 Problem Review #8) Phenylalanine is an essential amino acid whose chemical composition is 65. 5% C, 6. 67% H, 8. 48% N and 19. 4% O. What is the empirical formula ?

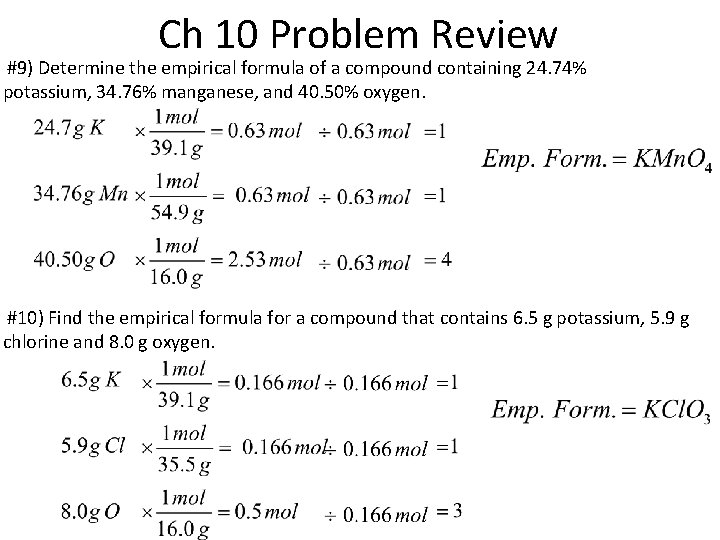
Ch 10 Problem Review #9) Determine the empirical formula of a compound containing 24. 74% potassium, 34. 76% manganese, and 40. 50% oxygen. #10) Find the empirical formula for a compound that contains 6. 5 g potassium, 5. 9 g chlorine and 8. 0 g oxygen.

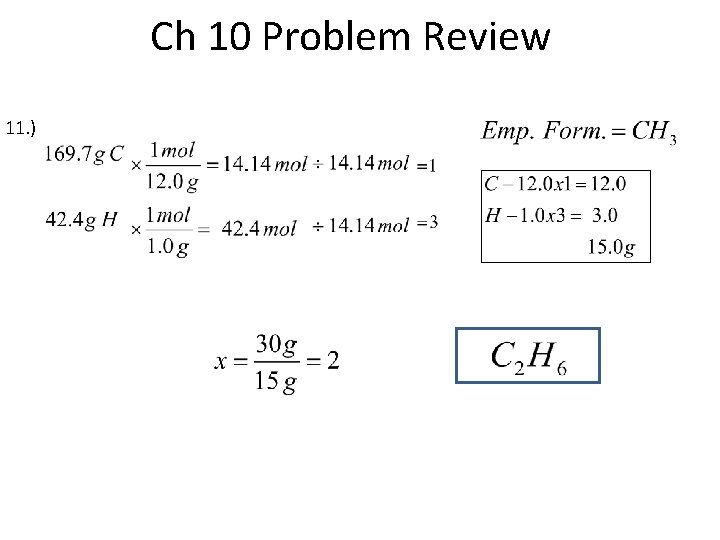
Ch 10 Problem Review 11. )

Chapter 10 Review What is a mole? -The number of atoms of an element equal to the number of atoms in exactly 12. 0 g of carbon-12. (That number happens to be 6. 02 x 1023) Why do we use the mole? -To simplify very large numbers Avagadro’s number? 6. 02 x 1023 – It is the number of particles in a mole of any substance.
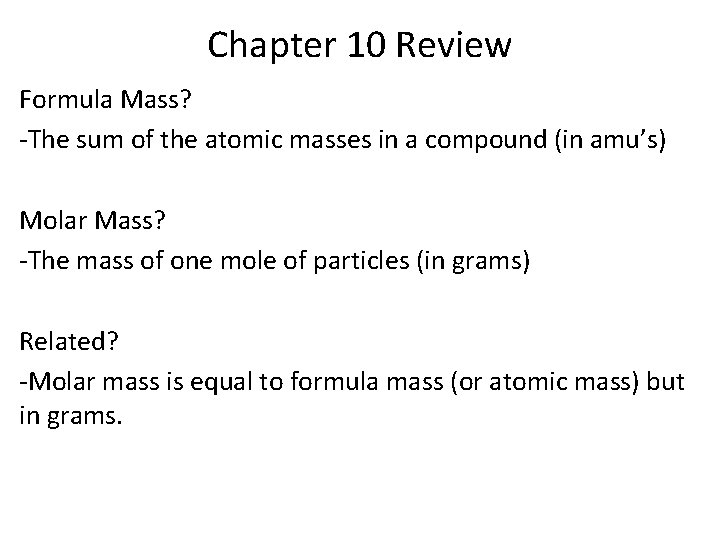
Chapter 10 Review Formula Mass? -The sum of the atomic masses in a compound (in amu’s) Molar Mass? -The mass of one mole of particles (in grams) Related? -Molar mass is equal to formula mass (or atomic mass) but in grams.

Chapter 10 Review Relationships -mass & moles: 1 mole = molar mass (g) -molecules/atoms/fu & moles: 1 mole = 6. 02 x 1023 particles -volume & moles: 1 mole = 22. 4 L Molar volume conditions? -STP (Standard Temp & Pressure) = 1 atm & 0°C

Chapter 10 Review Empirical Formula? -The simplest (reduced) formula (ratio of atoms) Molecular Formula? -Not reduced – so actual number of atoms present Related? -molecular formula = (empirical formula)x (molecular is empirical times some multiplier)

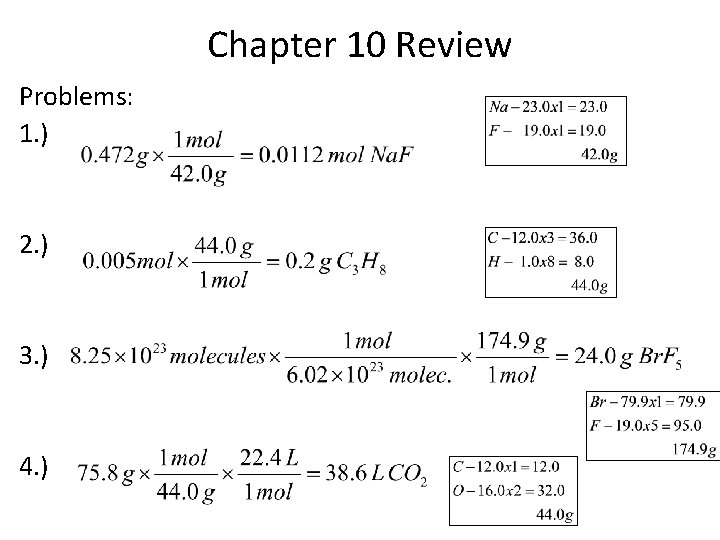
Chapter 10 Review Problems: 1. ) 2. ) 3. ) 4. )

Chapter 10 Review Problems: 5. ) Na – 23. 0 x 2 = 46. 0 C – 12. 0 x 2 = 24. 0 46. 0/134. 0 x 100 = 34. 3% Na 24. 0/134. 0 x 100 = 17. 9% C O – 16. 0 x 4 = 64. 0/134. 0 x 100 = 47. 8% O 134. 0 g 6. ) Copper(II)oxide = Cu. O Cu – 63. 5 x 1 = 63. 5/79. 5 x 100 = 79. 9% Cu O – 16. 0 x 1 = 16. 0 79. 5 g 0. 799 x 325 g = 259. 7 g

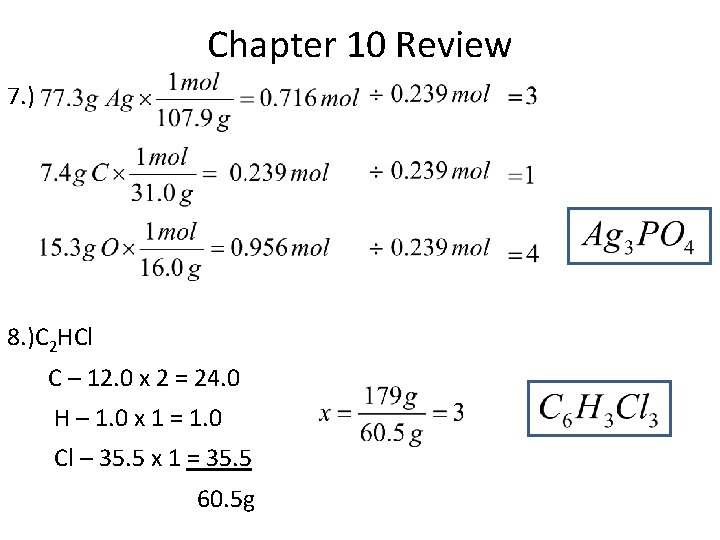
Chapter 10 Review 7. ) 8. )C 2 HCl C – 12. 0 x 2 = 24. 0 H – 1. 0 x 1 = 1. 0 Cl – 35. 5 x 1 = 35. 5 60. 5 g

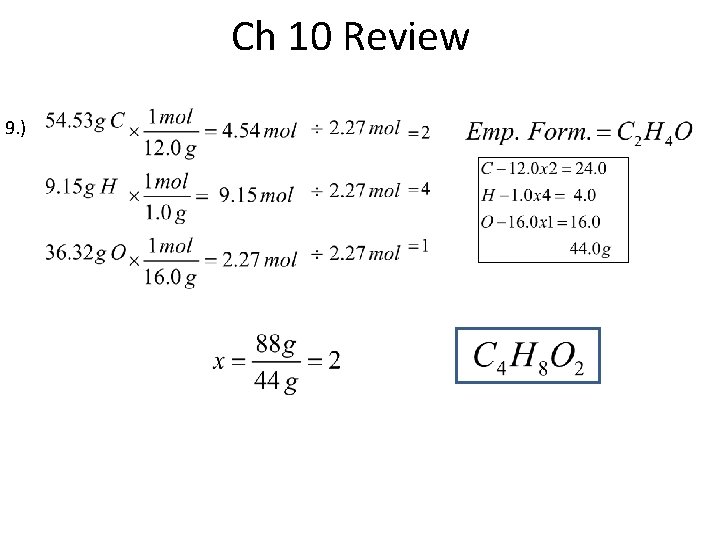
Ch 10 Review 9. )

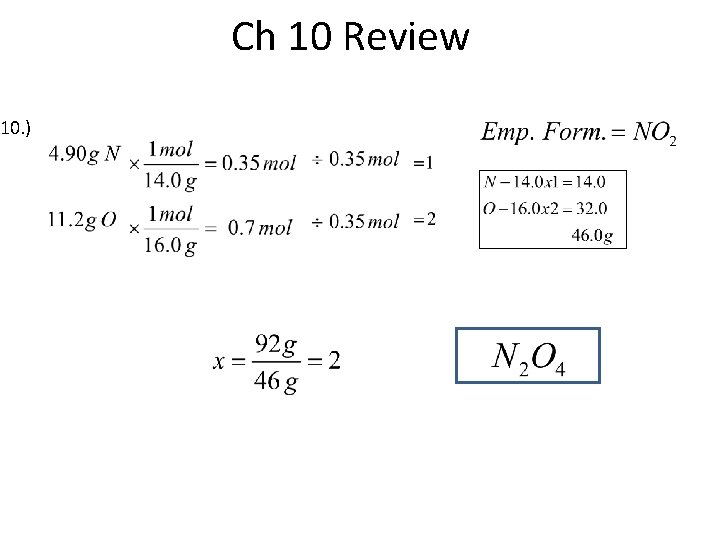
Ch 10 Review 10. )

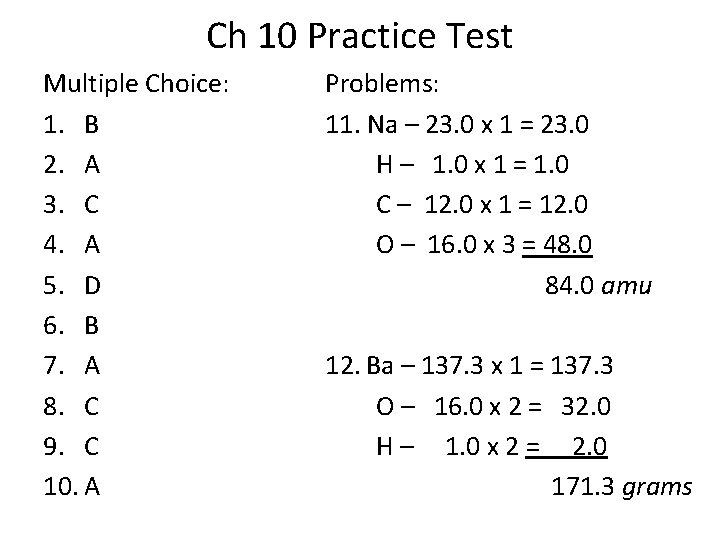
Ch 10 Practice Test Multiple Choice: 1. B 2. A 3. C 4. A 5. D 6. B 7. A 8. C 9. C 10. A Problems: 11. Na – 23. 0 x 1 = 23. 0 H – 1. 0 x 1 = 1. 0 C – 12. 0 x 1 = 12. 0 O – 16. 0 x 3 = 48. 0 84. 0 amu 12. Ba – 137. 3 x 1 = 137. 3 O – 16. 0 x 2 = 32. 0 H – 1. 0 x 2 = 2. 0 171. 3 grams

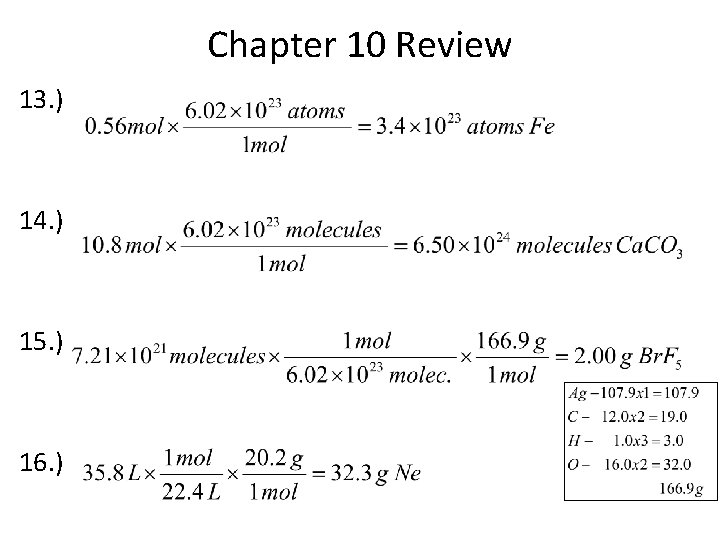
Chapter 10 Review 13. ) 14. ) 15. ) 16. )

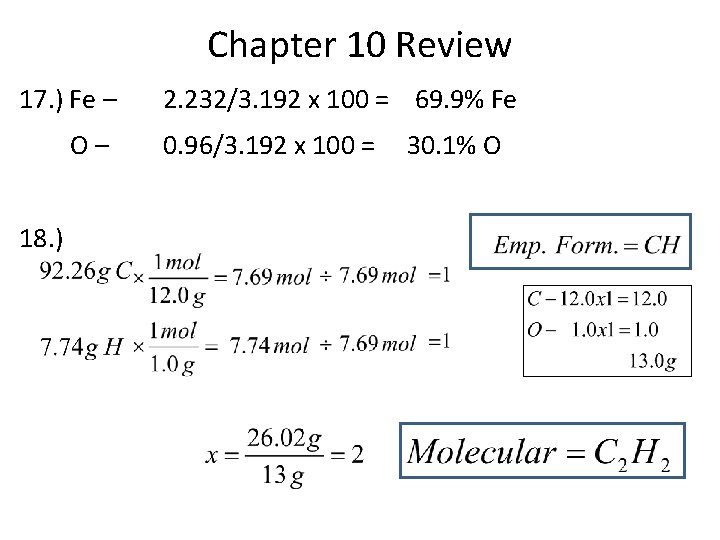
Chapter 10 Review 17. ) Fe – O– 18. ) 2. 232/3. 192 x 100 = 69. 9% Fe 0. 96/3. 192 x 100 = 30. 1% O

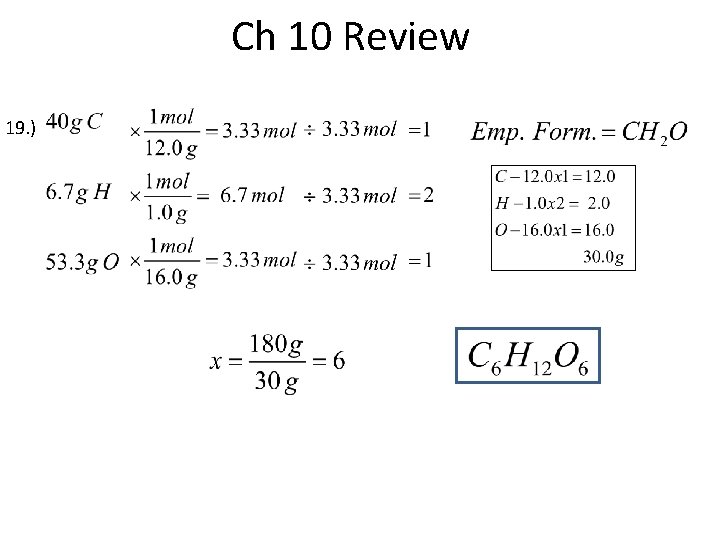
Ch 10 Review 19. )

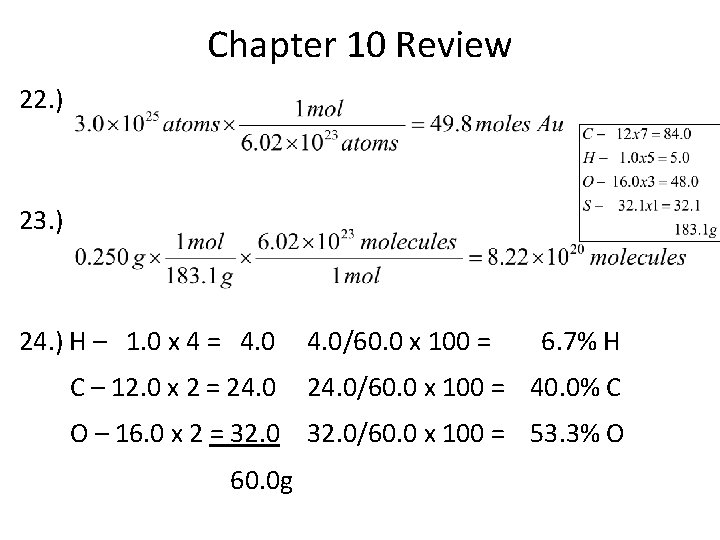
Chapter 10 Review 22. ) 23. ) 24. ) H – 1. 0 x 4 = 4. 0 C – 12. 0 x 2 = 24. 0/60. 0 x 100 = 6. 7% H 24. 0/60. 0 x 100 = 40. 0% C O – 16. 0 x 2 = 32. 0/60. 0 x 100 = 53. 3% O 60. 0 g