MassMass HW 7 2 Grams 1 Moles 2

- Slides: 13

Mass-Mass HW 7 -2 Grams 1 → Moles 2 → Grams 2

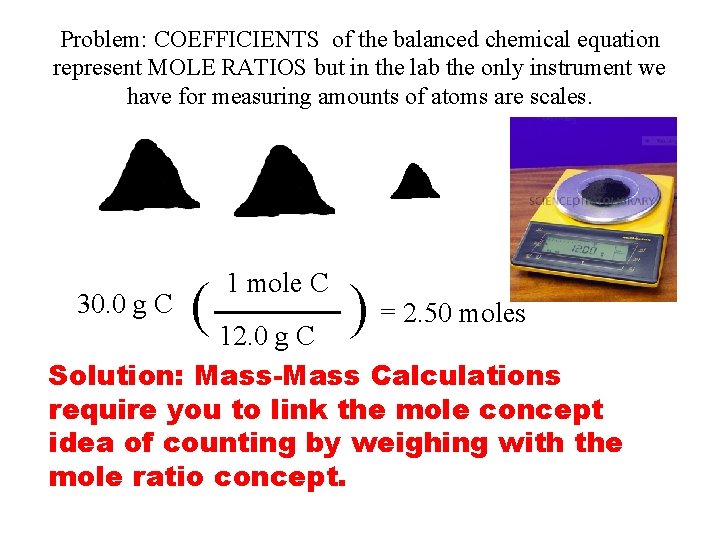
Problem: COEFFICIENTS of the balanced chemical equation represent MOLE RATIOS but in the lab the only instrument we have for measuring amounts of atoms are scales. 30. 0 g C 1 mole C ( 12. 0 g C ) = 2. 50 moles Solution: Mass-Mass Calculations require you to link the mole concept idea of counting by weighing with the mole ratio concept.

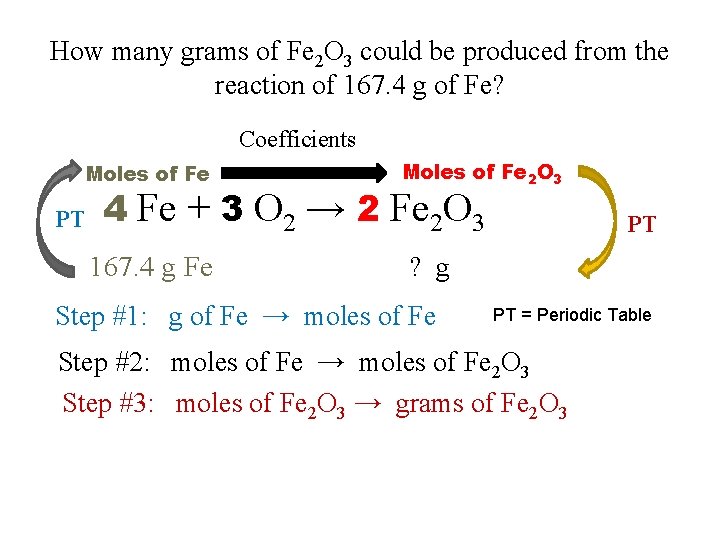
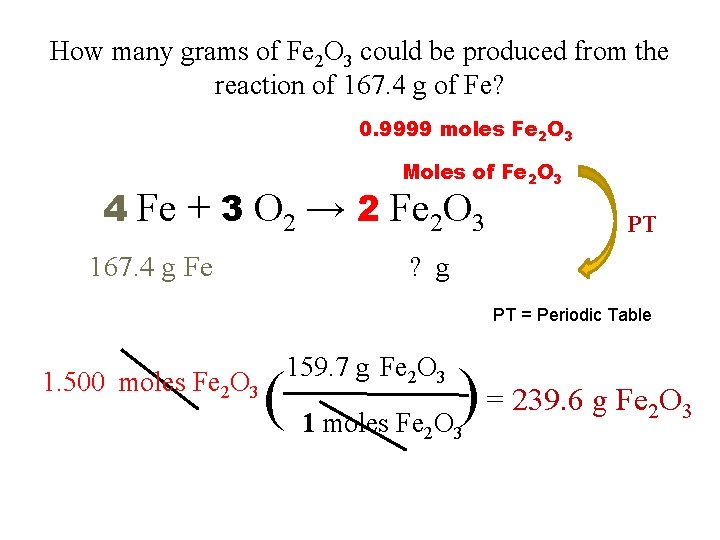
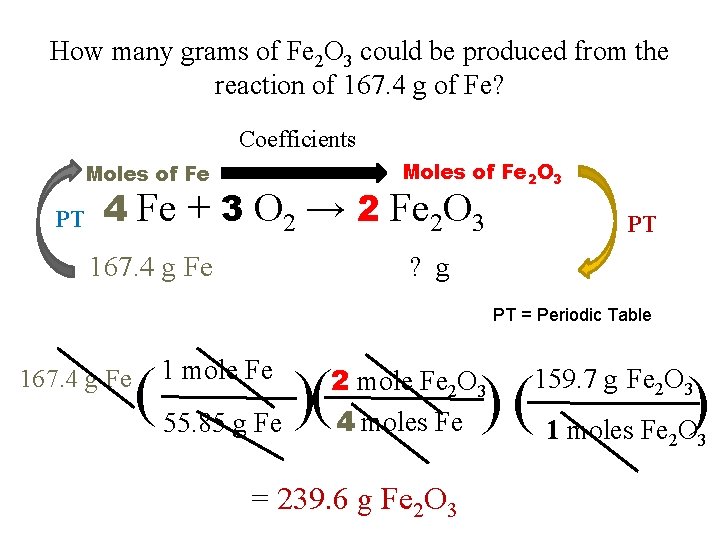
How many grams of Fe 2 O 3 could be produced from the reaction of 167. 4 g of Fe? Coefficients Moles of Fe PT Moles of Fe 2 O 3 4 Fe + 3 O 2 → 2 Fe 2 O 3 167. 4 g Fe PT ? g Step #1: g of Fe → moles of Fe PT = Periodic Table Step #2: moles of Fe → moles of Fe 2 O 3 Step #3: moles of Fe 2 O 3 → grams of Fe 2 O 3

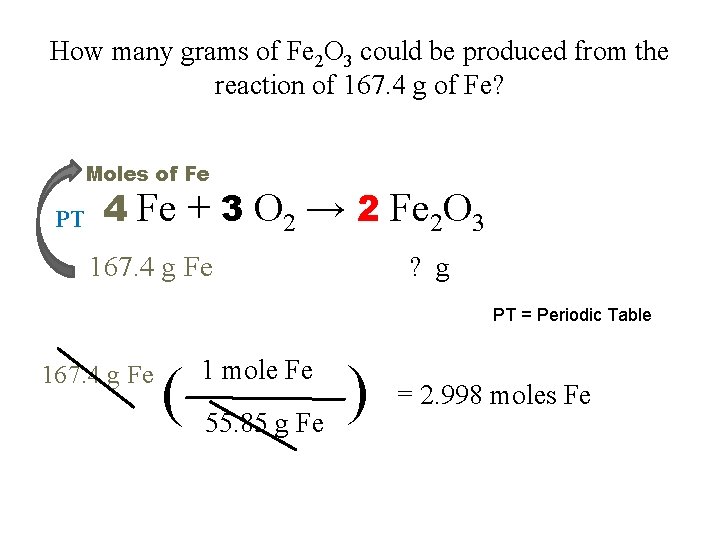
How many grams of Fe 2 O 3 could be produced from the reaction of 167. 4 g of Fe? Moles of Fe PT 4 Fe + 3 O 2 → 2 Fe 2 O 3 167. 4 g Fe ? g PT = Periodic Table 167. 4 g Fe ( 1 mole Fe 55. 85 g Fe ) = 2. 998 moles Fe

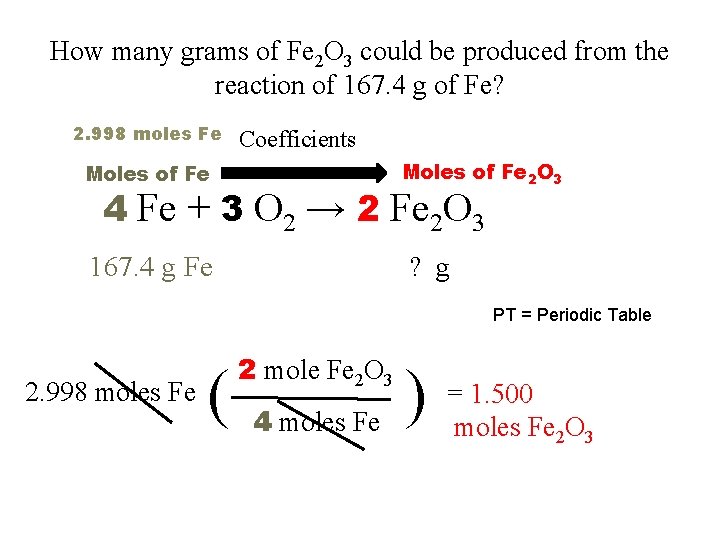
How many grams of Fe 2 O 3 could be produced from the reaction of 167. 4 g of Fe? 2. 998 moles Fe Coefficients Moles of Fe 2 O 3 Moles of Fe 4 Fe + 3 O 2 → 2 Fe 2 O 3 167. 4 g Fe ? g PT = Periodic Table 2. 998 moles Fe ( 2 mole Fe 2 O 3 4 moles Fe ) = 1. 500 moles Fe 2 O 3

How many grams of Fe 2 O 3 could be produced from the reaction of 167. 4 g of Fe? 0. 9999 moles Fe 2 O 3 Moles of Fe 2 O 3 4 Fe + 3 O 2 → 2 Fe 2 O 3 167. 4 g Fe PT ? g PT = Periodic Table 1. 500 moles Fe 2 O 3 159. 7 g Fe 2 O 3 ( 1 moles Fe O ) = 239. 6 g Fe O 2 3

How many grams of Fe 2 O 3 could be produced from the reaction of 167. 4 g of Fe? Coefficients Moles of Fe 2 O 3 Moles of Fe PT 4 Fe + 3 O 2 → 2 Fe 2 O 3 167. 4 g Fe PT ? g PT = Periodic Table 167. 4 g Fe 1 mole Fe ( 55. 85 g Fe )( 2 mole Fe 2 O 3 4 moles Fe = 239. 6 g Fe 2 O 3 ) ( 1 moles Fe O) 159. 7 g Fe 2 O 3 2 3

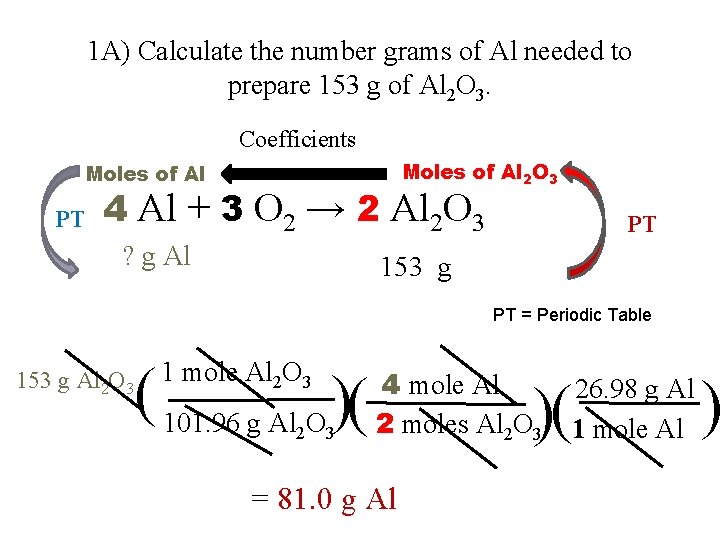
1 A) Calculate the number grams of Al needed to prepare 153 g of Al 2 O 3. Coefficients Moles of Al 2 O 3 Moles of Al PT 4 Al + 3 O 2 → 2 Al 2 O 3 ? g Al PT 153 g PT = Periodic Table 153 g Al 2 O 3 ( 101. 96 g Al O )( 1 mole Al 2 O 3 2 3 4 mole Al 2 moles Al 2 O 3 = 81. 0 g Al )( 26. 98 g Al 1 mole Al )

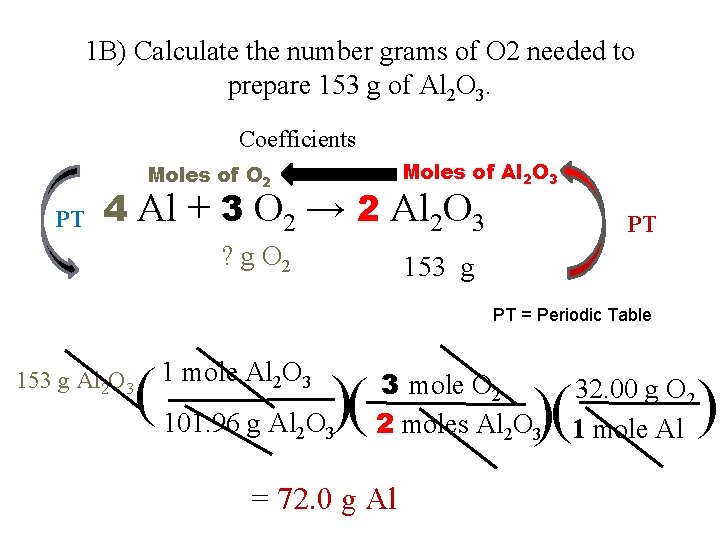
1 B) Calculate the number grams of O 2 needed to prepare 153 g of Al 2 O 3. Coefficients Moles of Al 2 O 3 Moles of O 2 PT 4 Al + 3 O 2 → 2 Al 2 O 3 ? g O 2 PT 153 g PT = Periodic Table 153 g Al 2 O 3 ( 101. 96 g Al O )( 1 mole Al 2 O 3 2 3 3 mole O 2 2 moles Al 2 O 3 = 72. 0 g Al )( ) 32. 00 g O 2 1 mole Al

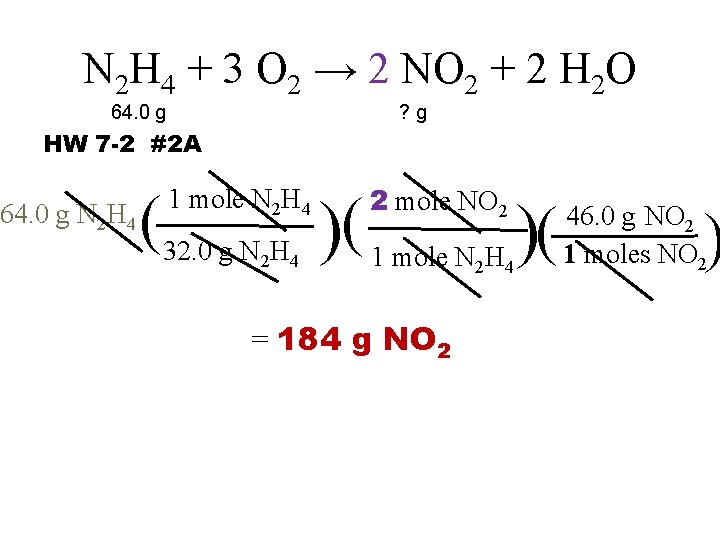
N 2 H 4 + 3 O 2 → 2 NO 2 + 2 H 2 O 64. 0 g ? g HW 7 -2 #2 A 64. 0 g N 2 H 4 2 mole NO 2 (32. 0 g N H )( 1 mole N 2 H 4 2 4 = 184 g NO 2 2 4 ) 46. 0 g NO 2 1 moles NO 2

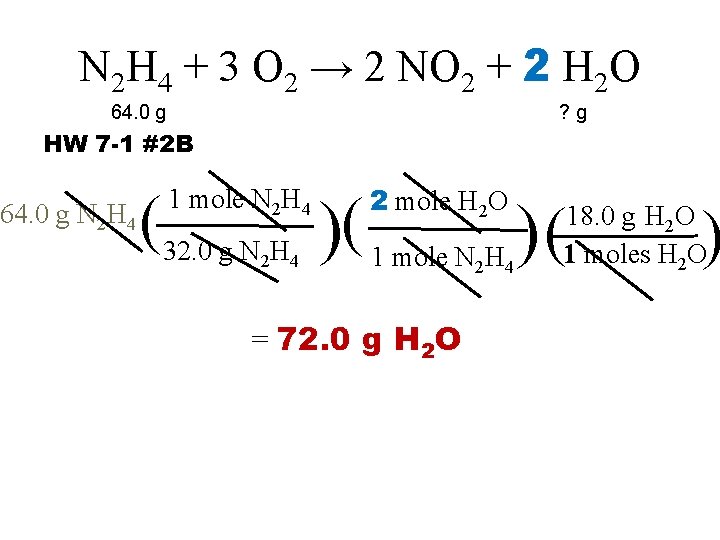
N 2 H 4 + 3 O 2 → 2 NO 2 + 2 H 2 O 64. 0 g ? g HW 7 -1 #2 B 64. 0 g N 2 H 4 2 mole H 2 O (32. 0 g N H )( 1 mole N H ) ( 1 mole N 2 H 4 2 4 = 72. 0 g H 2 O 2 4 ) 18. 0 g H 2 O 1 moles H 2 O

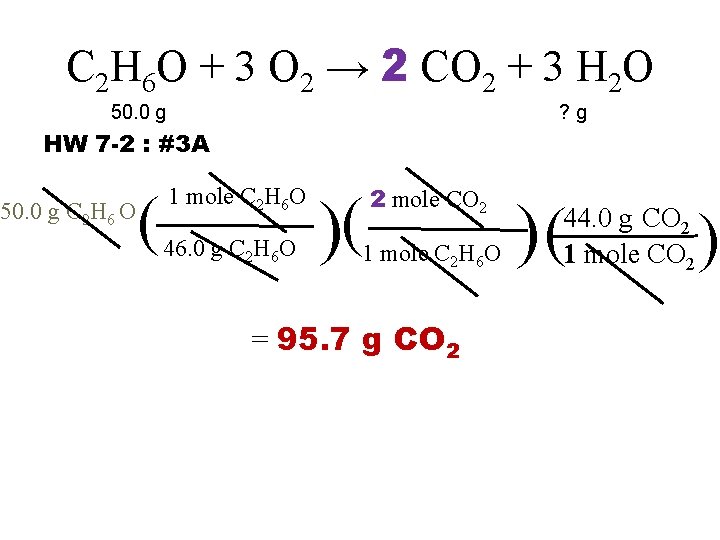
C 2 H 6 O + 3 O 2 → 2 CO 2 + 3 H 2 O 50. 0 g ? g HW 7 -2 : #3 A 50. 0 g C 2 H 6 O ( 1 mole C 2 H 6 O 46. 0 g C 2 H 6 O )( 2 mole CO 2 1 mole C 2 H 6 O = 95. 7 g CO 2 )( 44. 0 g CO 2 1 mole CO 2 )

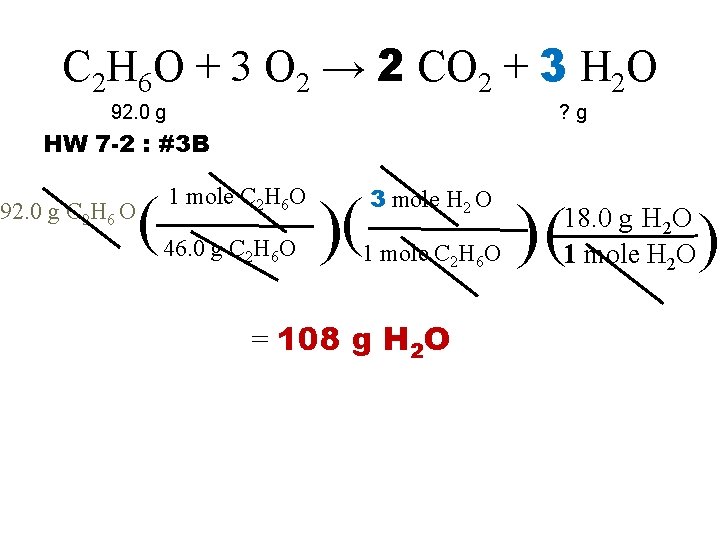
C 2 H 6 O + 3 O 2 → 2 CO 2 + 3 H 2 O 92. 0 g ? g HW 7 -2 : #3 B 92. 0 g C 2 H 6 O ( 1 mole C 2 H 6 O 46. 0 g C 2 H 6 O )( 3 mole H 2 O 1 mole C 2 H 6 O = 108 g H 2 O )( 18. 0 g H 2 O 1 mole H 2 O )
 Kno3 oxidation number of n
Kno3 oxidation number of n Mass to moles conversion factor
Mass to moles conversion factor Grams to moles
Grams to moles You have 23 moles of tantalum (ta). how many grams is this
You have 23 moles of tantalum (ta). how many grams is this How to turn grams into moles
How to turn grams into moles Mols to grams
Mols to grams Atoms to grams
Atoms to grams Mass to moles
Mass to moles Molar solution
Molar solution Mole road map chemistry
Mole road map chemistry Dimensional analysis grams to moles
Dimensional analysis grams to moles Grams to mols
Grams to mols How to find molecular concentration
How to find molecular concentration Moles to grams chart
Moles to grams chart