Chapter 9 Stoichiometry Converting grams to moles same


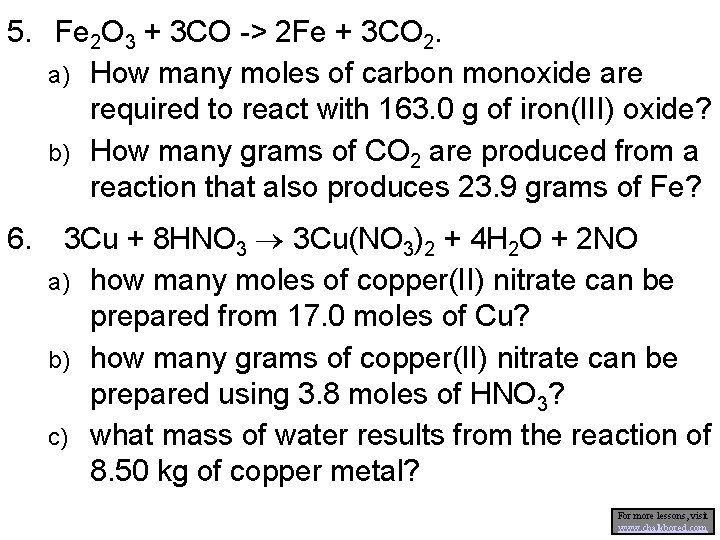





- Slides: 39

Chapter 9 Stoichiometry

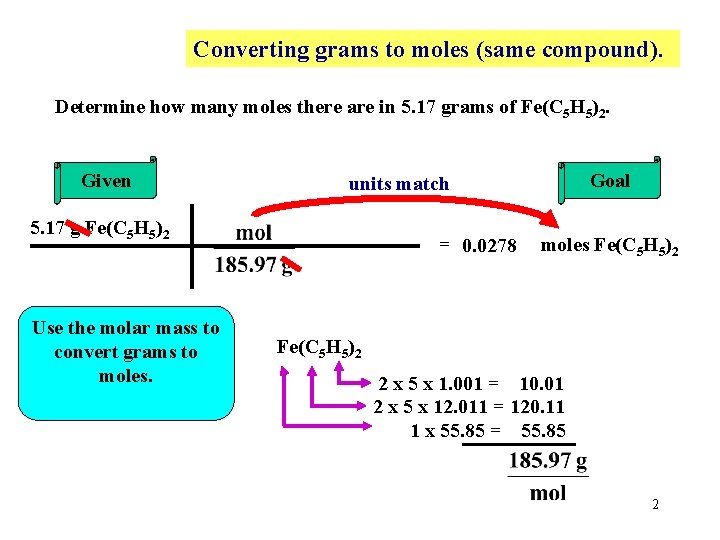
Converting grams to moles (same compound). Determine how many moles there are in 5. 17 grams of Fe(C 5 H 5)2. Given 5. 17 g Fe(C 5 H 5)2 Use the molar mass to convert grams to moles. Goal units match = 0. 0278 moles Fe(C 5 H 5)2 2 x 5 x 1. 001 = 10. 01 2 x 5 x 12. 011 = 120. 11 1 x 55. 85 = 55. 85 2

What is Stoichiometry? • It is the calculation of the quantitative relationships in chemical equations. • A recipe for chemical equations. • Shows how mass and matter are conserved in reactions

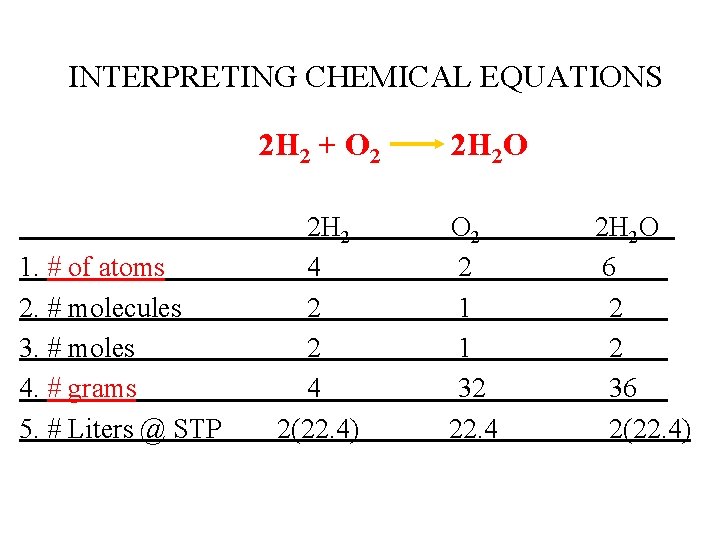
INTERPRETING CHEMICAL EQUATIONS 2 H 2 + O 2 1. # of atoms 2. # molecules 3. # moles 4. # grams 5. # Liters @ STP 2 H 2 4 2(22. 4) 2 H 2 O O 2 2 1 1 32 22. 4 2 H 2 O 6 2 2 36 2(22. 4)

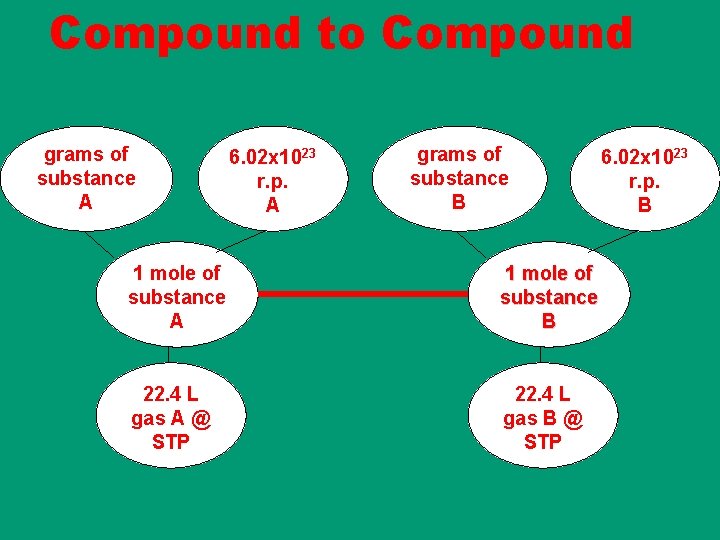
Compound to Compound grams of substance A 6. 02 x 1023 r. p. A grams of substance B 1 mole of substance A 1 mole of substance B 22. 4 L gas A @ STP 22. 4 L gas B @ STP 6. 02 x 1023 r. p. B

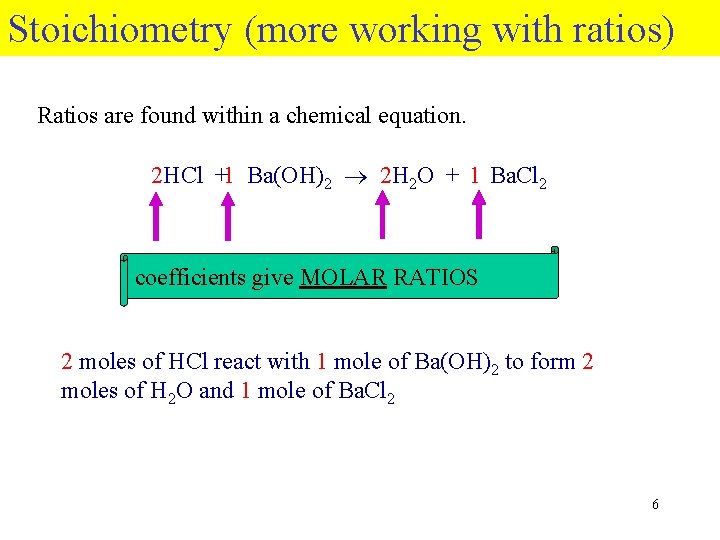
Stoichiometry (more working with ratios) Ratios are found within a chemical equation. 2 HCl +1 Ba(OH)2 2 H 2 O + 1 Ba. Cl 2 coefficients give MOLAR RATIOS 2 moles of HCl react with 1 mole of Ba(OH)2 to form 2 moles of H 2 O and 1 mole of Ba. Cl 2 6

Stoichiometry • • Recall also that molar masses provide factors: 1 mol NH 3 / 17 g NH 3, 32 g O 2 / 1 mol O 2 • • Consider: 4 NH 3 + 5 O 2 6 H 2 O + 4 NO many conversion factors exist: 4 mol NH 3/5 mol O 2, 6 mol H 2 O/4 mol NH 3, etc • refers to calculations that make use of mole ratios.

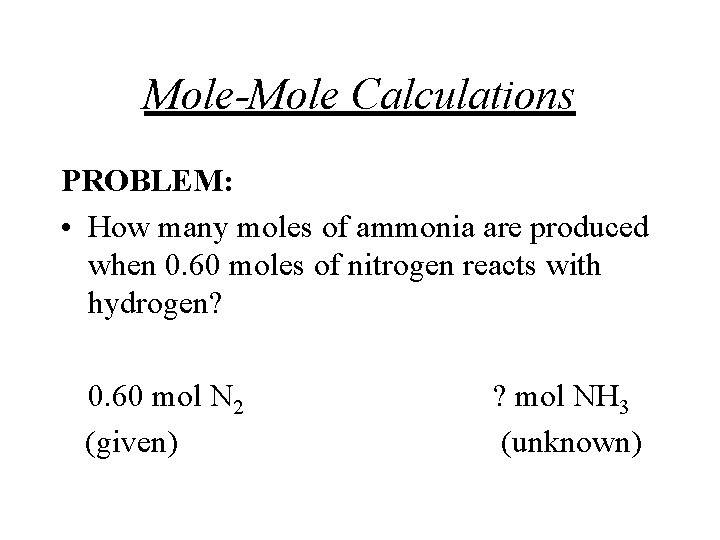
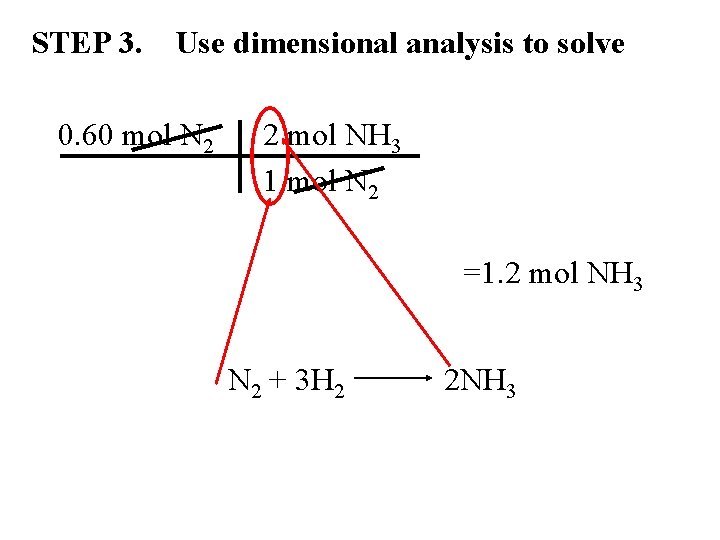
Mole-Mole Calculations PROBLEM: • How many moles of ammonia are produced when 0. 60 moles of nitrogen reacts with hydrogen? 0. 60 mol N 2 (given) ? mol NH 3 (unknown)

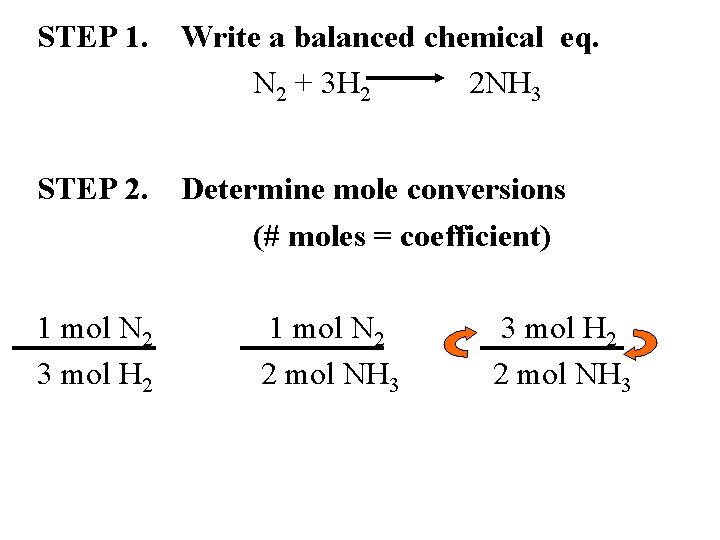
STEP 1. Write a balanced chemical eq. N 2 + 3 H 2 2 NH 3 STEP 2. Determine mole conversions (# moles = coefficient) 1 mol N 2 3 mol H 2 1 mol N 2 2 mol NH 3 3 mol H 2 2 mol NH 3

STEP 3. Use dimensional analysis to solve 0. 60 mol N 2 2 mol NH 3 1 mol N 2 =1. 2 mol NH 3 N 2 + 3 H 2 2 NH 3

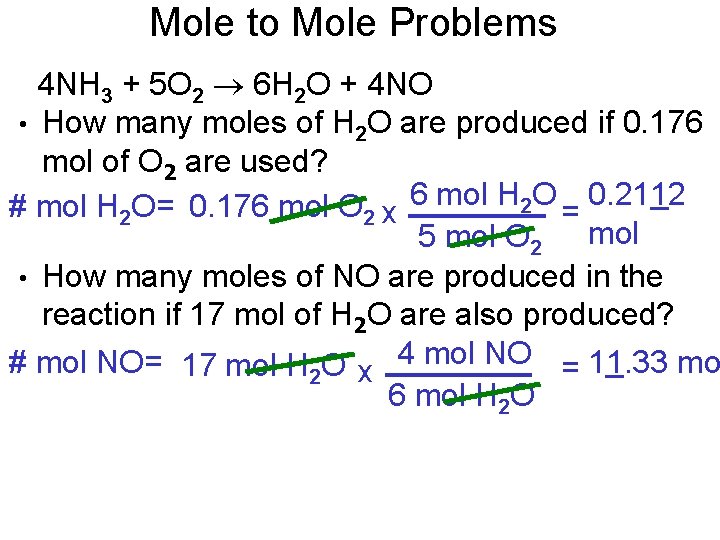
Mole to Mole Problems 4 NH 3 + 5 O 2 6 H 2 O + 4 NO • How many moles of H 2 O are produced if 0. 176 mol of O 2 are used? # mol H 2 O= 0. 176 mol O 2 x 6 mol H 2 O = 0. 2112 5 mol O 2 mol • How many moles of NO are produced in the reaction if 17 mol of H 2 O are also produced? # mol NO= 17 mol H 2 O x 4 mol NO = 11. 33 mo 6 mol H 2 O

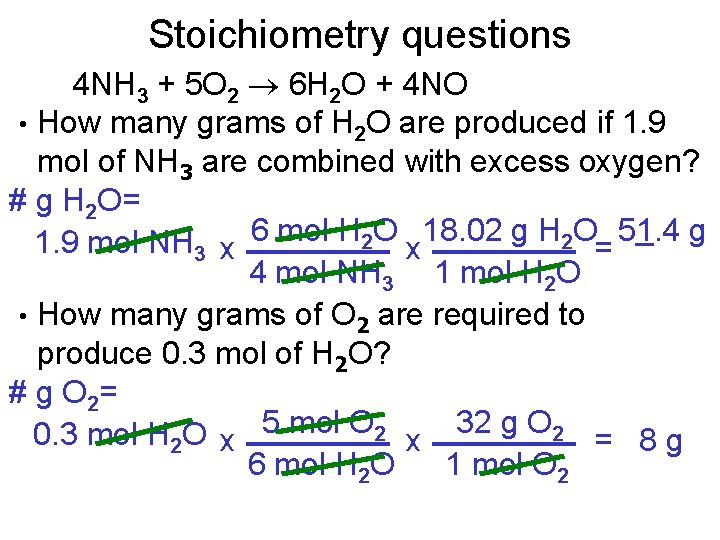
Stoichiometry questions 4 NH 3 + 5 O 2 6 H 2 O + 4 NO • How many grams of H 2 O are produced if 1. 9 mol of NH 3 are combined with excess oxygen? # g H 2 O= 1. 9 mol NH 3 x 6 mol H 2 O x 18. 02 g H 2 O= 51. 4 g 4 mol NH 3 1 mol H 2 O • How many grams of O 2 are required to produce 0. 3 mol of H 2 O? # g O 2= 0. 3 mol H 2 O x 5 mol O 2 x 32 g O 2 = 8 g 6 mol H 2 O 1 mol O 2

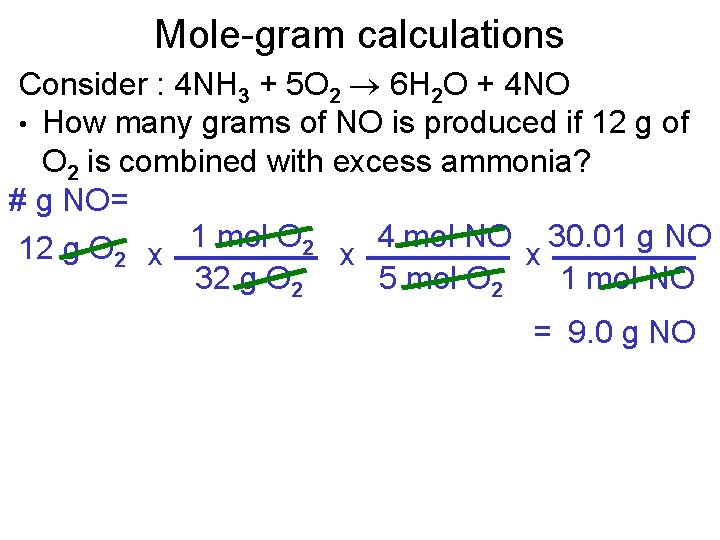
Mole-gram calculations Consider : 4 NH 3 + 5 O 2 6 H 2 O + 4 NO • How many grams of NO is produced if 12 g of O 2 is combined with excess ammonia? # g NO= 12 g O 2 x 1 mol O 2 x 4 mol NO x 30. 01 g NO 32 g O 2 5 mol O 2 1 mol NO = 9. 0 g NO

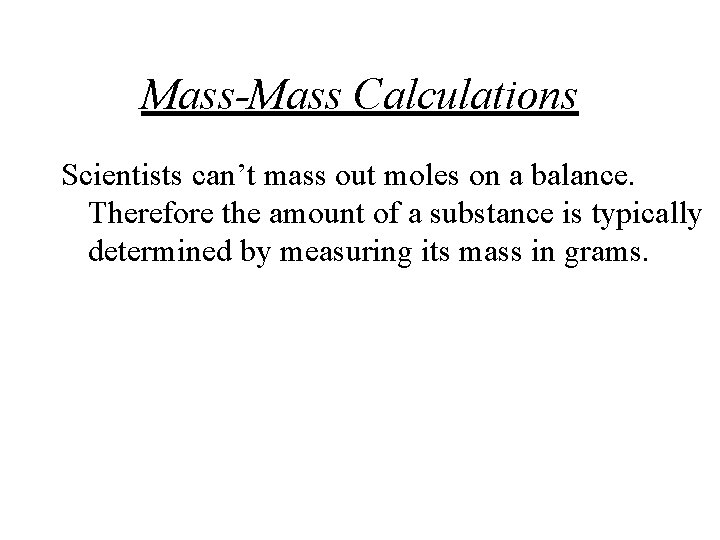
Mass-Mass Calculations Scientists can’t mass out moles on a balance. Therefore the amount of a substance is typically determined by measuring its mass in grams.

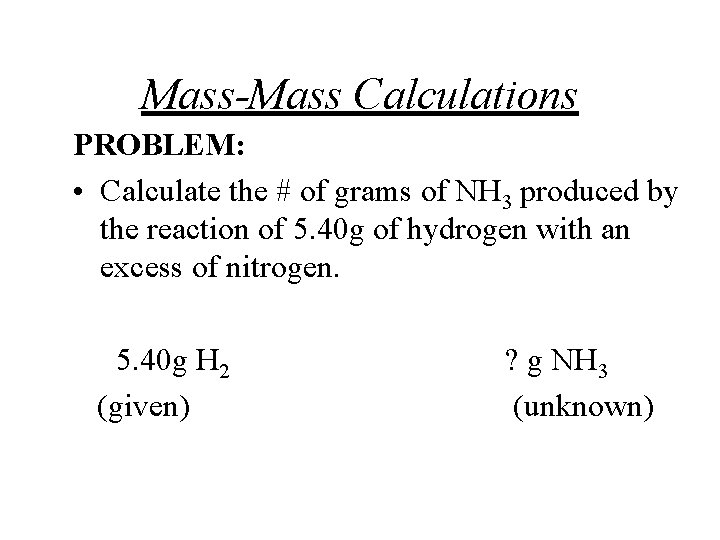
Mass-Mass Calculations PROBLEM: • Calculate the # of grams of NH 3 produced by the reaction of 5. 40 g of hydrogen with an excess of nitrogen. 5. 40 g H 2 (given) ? g NH 3 (unknown)

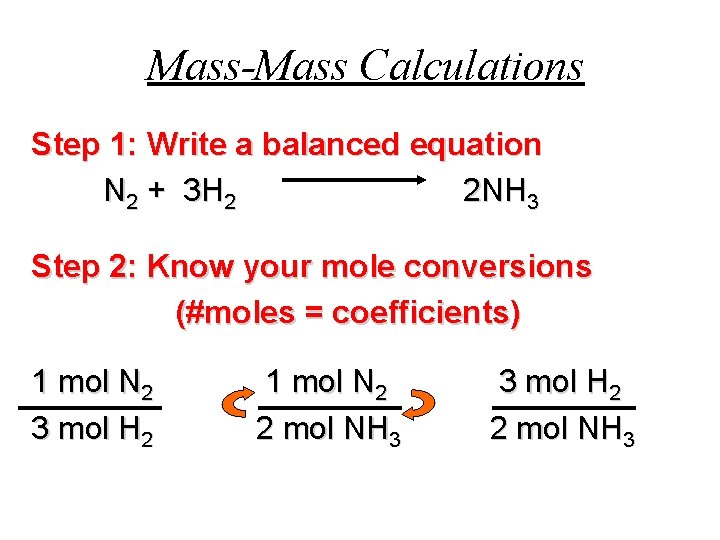
Mass-Mass Calculations Step 1: Write a balanced equation N 2 + 3 H 2 2 NH 3 Step 2: Know your mole conversions (#moles = coefficients) 1 mol N 2 3 mol H 2 1 mol N 2 2 mol NH 3 3 mol H 2 2 mol NH 3

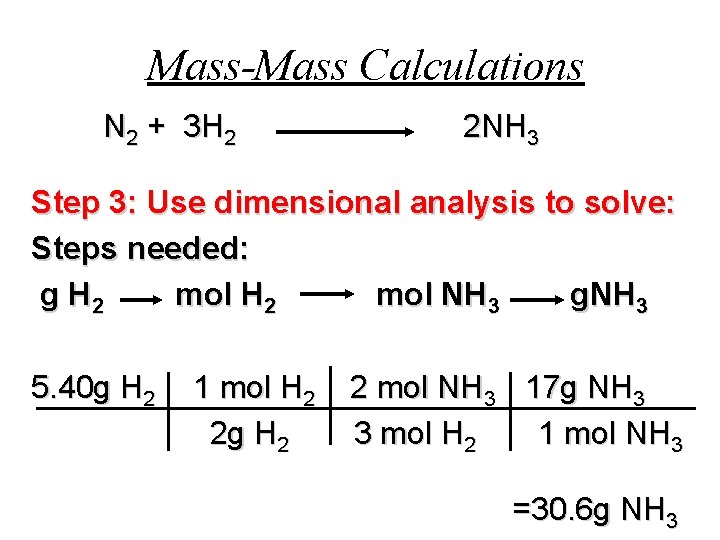
Mass-Mass Calculations N 2 + 3 H 2 2 NH 3 Step 3: Use dimensional analysis to solve: Steps needed: g H 2 mol NH 3 g. NH 3 5. 40 g H 2 1 mol H 2 2 g H 2 2 mol NH 3 17 g NH 3 3 mol H 2 1 mol NH 3 =30. 6 g NH 3

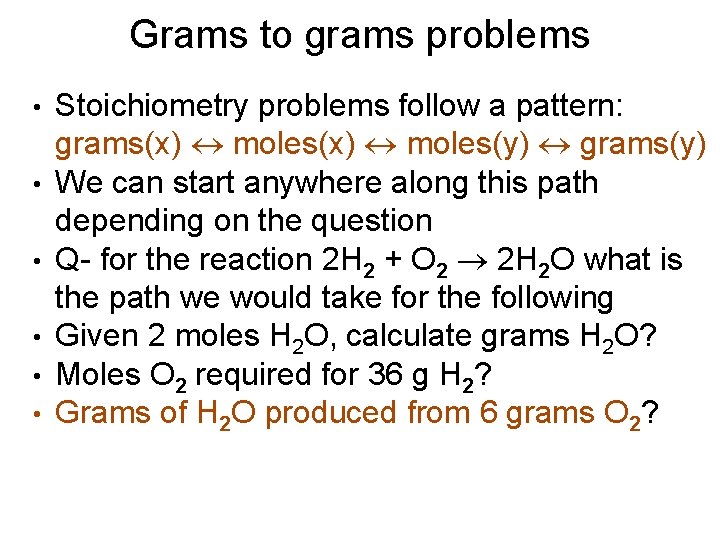
Grams to grams problems • • • Stoichiometry problems follow a pattern: grams(x) moles(y) grams(y) We can start anywhere along this path depending on the question Q- for the reaction 2 H 2 + O 2 2 H 2 O what is the path we would take for the following Given 2 moles H 2 O, calculate grams H 2 O? Moles O 2 required for 36 g H 2? Grams of H 2 O produced from 6 grams O 2?

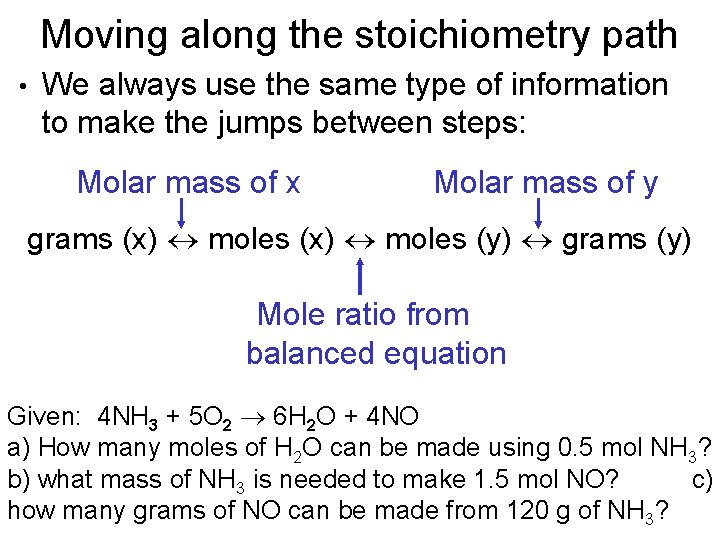
Moving along the stoichiometry path • We always use the same type of information to make the jumps between steps: Molar mass of x Molar mass of y grams (x) moles (y) grams (y) Mole ratio from balanced equation Given: 4 NH 3 + 5 O 2 6 H 2 O + 4 NO a) How many moles of H 2 O can be made using 0. 5 mol NH 3? b) what mass of NH 3 is needed to make 1. 5 mol NO? c) how many grams of NO can be made from 120 g of NH 3?

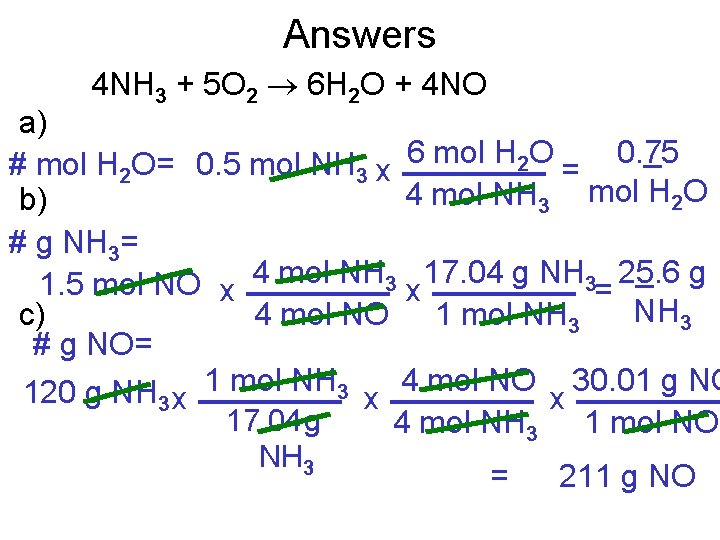
Answers 4 NH 3 + 5 O 2 6 H 2 O + 4 NO a) # mol H 2 O= 0. 5 mol NH 3 x 6 mol H 2 O = 0. 75 4 mol NH 3 mol H 2 O b) # g NH 3= 1. 5 mol NO x 4 mol NH 3 x 17. 04 g NH 3= 25. 6 g NH 3 4 mol NO 1 mol NH 3 c) # g NO= 120 g NH 3 x 1 mol NH 3 x 4 mol NO x 30. 01 g NO 17. 04 g 4 mol NH 3 1 mol NO NH 3 = 211 g NO

More Stoichiometry Questions Follow the rules for significant digits. Show all calculations. 1. 2 C 4 H 10 + 13 O 2 -> 8 CO 2 + 10 H 2 O a) what mass of O 2 will react with 400 g C 4 H 10? b) how many moles of water are formed in a)? 2. 3 HCl + Al(OH)3 -> 3 H 2 O + Al. Cl 3 How many grams of aluminum hydroxide will react with 5. 3 moles of HCl? 3. Ca(Cl. O 3)2 -> Ca. Cl 2 + 3 O 2 What mass of O 2 results from the decomposition of 1. 00 kg of calcium chlorate? 4. The reaction of Ca with water can be predicted using the activity series. What mass of water is needed to completely react with 2. 35 g of Ca?
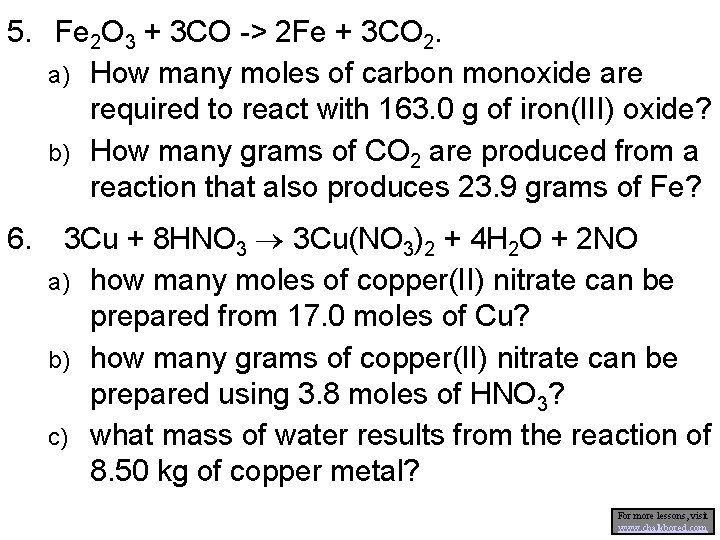
5. Fe 2 O 3 + 3 CO -> 2 Fe + 3 CO 2. a) How many moles of carbon monoxide are required to react with 163. 0 g of iron(III) oxide? b) How many grams of CO 2 are produced from a reaction that also produces 23. 9 grams of Fe? 6. 3 Cu + 8 HNO 3 3 Cu(NO 3)2 + 4 H 2 O + 2 NO a) how many moles of copper(II) nitrate can be prepared from 17. 0 moles of Cu? b) how many grams of copper(II) nitrate can be prepared using 3. 8 moles of HNO 3? c) what mass of water results from the reaction of 8. 50 kg of copper metal? For more lessons, visit www. chalkbored. com

9 -3 Limiting Reactants and Percent Yield

Limiting Reactant A limiting reactant in a chemical reaction is the substance that • Is used up first. • Stops the reaction. • Limits the amount of product that can form. 24

Reacting Amounts In a table setting, there is 1 plate, 1 fork, 1 knife, and 1 spoon. How many table settings are possible from 5 plates, 6 forks, 4 spoons, and 7 knives? What is the limiting item? 25 Copyright © 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings

Reacting Amounts Four table settings can be made. Initially Use Left over plates 5 4 1 forks 6 4 2 spoons 4 4 0 knives 7 4 3 The limiting item is the spoon. 26

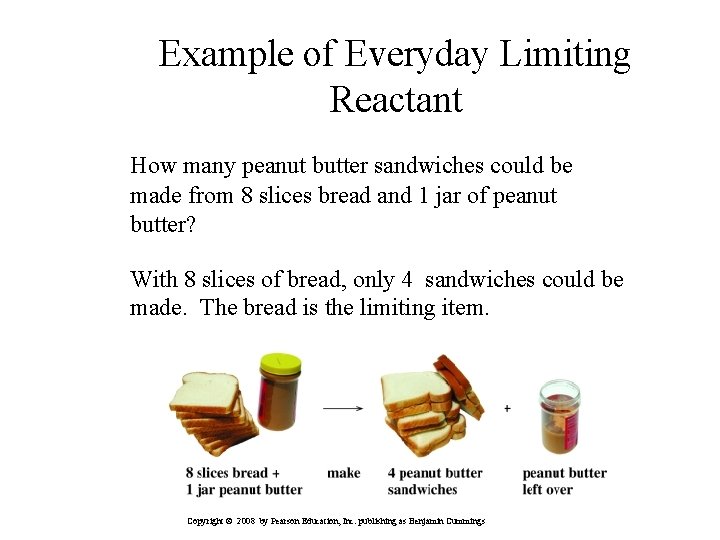
Example of Everyday Limiting Reactant How many peanut butter sandwiches could be made from 8 slices bread and 1 jar of peanut butter? With 8 slices of bread, only 4 sandwiches could be made. The bread is the limiting item. 27 Copyright © 2008 by Pearson Education, Inc. publishing as Benjamin Cummings

Example of Everyday Limiting Reactant How many peanut butter sandwiches could be made from 8 slices bread and 1 tablespoon of peanut butter? With 1 tablespoon of peanut butter, only 1 sandwich could be made. The peanut butter is the limiting item. Copyright © 2008 by Pearson Education, Inc. publishing as Benjamin Cummings 28

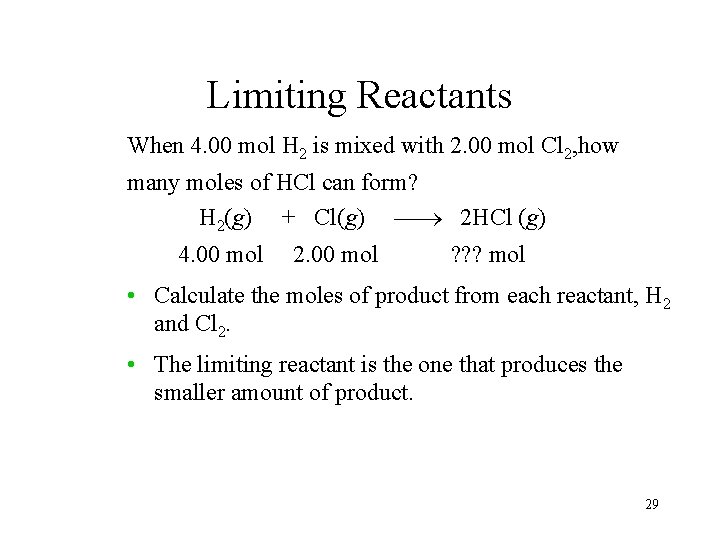
Limiting Reactants When 4. 00 mol H 2 is mixed with 2. 00 mol Cl 2, how many moles of HCl can form? H 2(g) + Cl(g) 2 HCl (g) 4. 00 mol 2. 00 mol ? ? ? mol • Calculate the moles of product from each reactant, H 2 and Cl 2. • The limiting reactant is the one that produces the smaller amount of product. 29

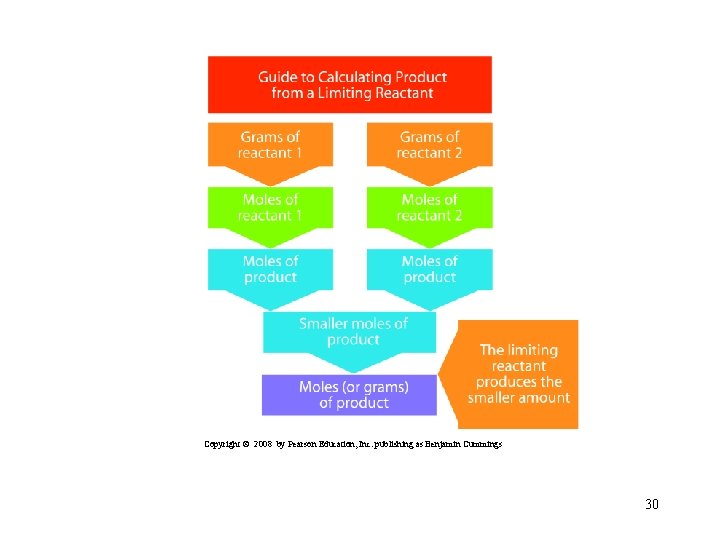
Copyright © 2008 by Pearson Education, Inc. publishing as Benjamin Cummings 30

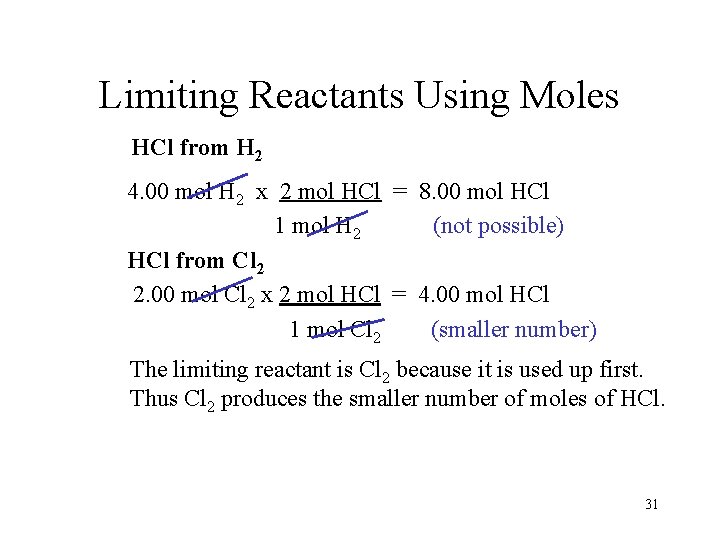
Limiting Reactants Using Moles HCl from H 2 4. 00 mol H 2 x 2 mol HCl = 8. 00 mol HCl 1 mol H 2 (not possible) HCl from Cl 2 2. 00 mol Cl 2 x 2 mol HCl = 4. 00 mol HCl 1 mol Cl 2 (smaller number) The limiting reactant is Cl 2 because it is used up first. Thus Cl 2 produces the smaller number of moles of HCl. 31

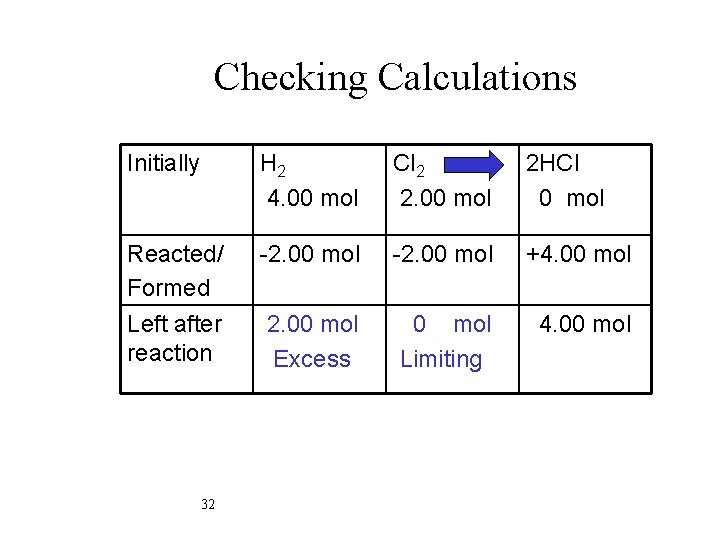
Checking Calculations Initially H 2 4. 00 mol Cl 2 2. 00 mol 2 HCl 0 mol Reacted/ Formed -2. 00 mol +4. 00 mol Left after reaction 2. 00 mol Excess 0 mol Limiting 4. 00 mol 32

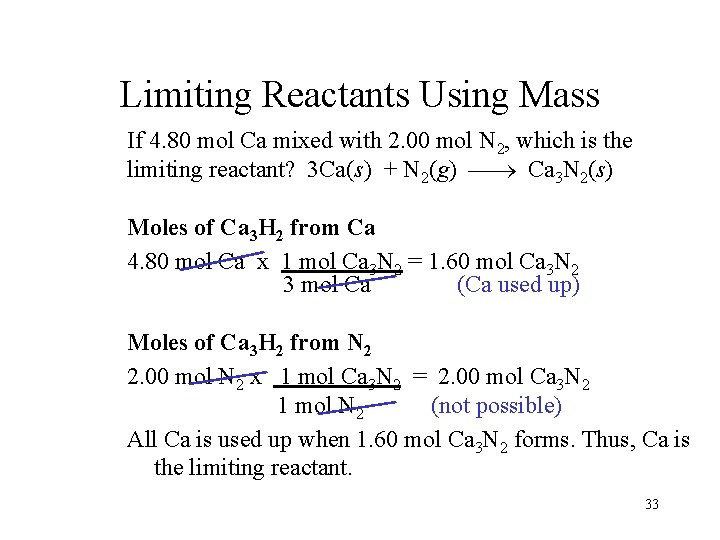
Limiting Reactants Using Mass If 4. 80 mol Ca mixed with 2. 00 mol N 2, which is the limiting reactant? 3 Ca(s) + N 2(g) Ca 3 N 2(s) Moles of Ca 3 H 2 from Ca 4. 80 mol Ca x 1 mol Ca 3 N 2 = 1. 60 mol Ca 3 N 2 3 mol Ca (Ca used up) Moles of Ca 3 H 2 from N 2 2. 00 mol N 2 x 1 mol Ca 3 N 2 = 2. 00 mol Ca 3 N 2 1 mol N 2 (not possible) All Ca is used up when 1. 60 mol Ca 3 N 2 forms. Thus, Ca is the limiting reactant. 33

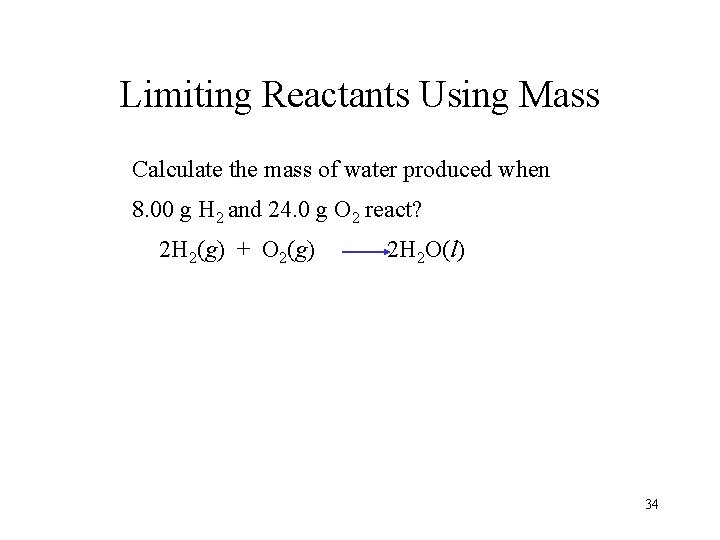
Limiting Reactants Using Mass Calculate the mass of water produced when 8. 00 g H 2 and 24. 0 g O 2 react? 2 H 2(g) + O 2(g) 2 H 2 O(l) 34

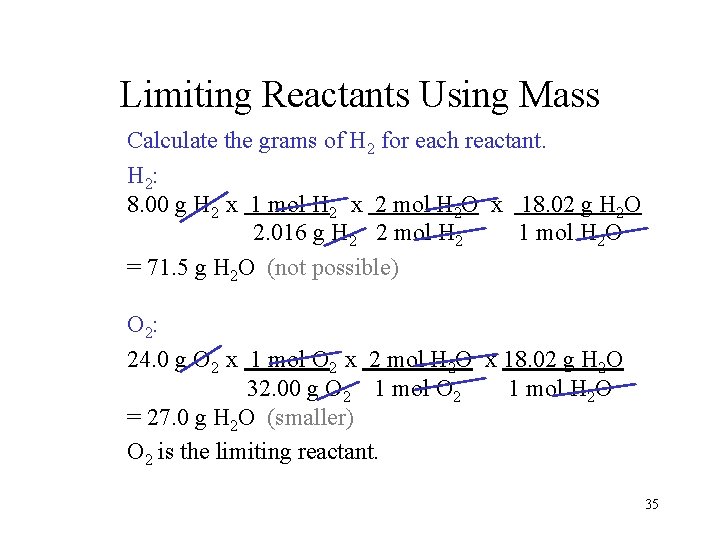
Limiting Reactants Using Mass Calculate the grams of H 2 for each reactant. H 2: 8. 00 g H 2 x 1 mol H 2 x 2 mol H 2 O x 18. 02 g H 2 O 2. 016 g H 2 2 mol H 2 1 mol H 2 O = 71. 5 g H 2 O (not possible) O 2: 24. 0 g O 2 x 1 mol O 2 x 2 mol H 2 O x 18. 02 g H 2 O 32. 00 g O 2 1 mol H 2 O = 27. 0 g H 2 O (smaller) O 2 is the limiting reactant. 35

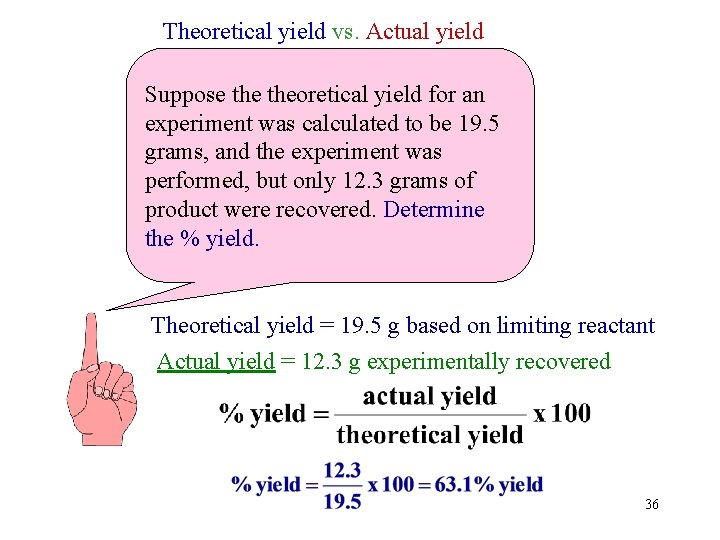
Theoretical yield vs. Actual yield Suppose theoretical yield for an experiment was calculated to be 19. 5 grams, and the experiment was performed, but only 12. 3 grams of product were recovered. Determine the % yield. Theoretical yield = 19. 5 g based on limiting reactant Actual yield = 12. 3 g experimentally recovered 36

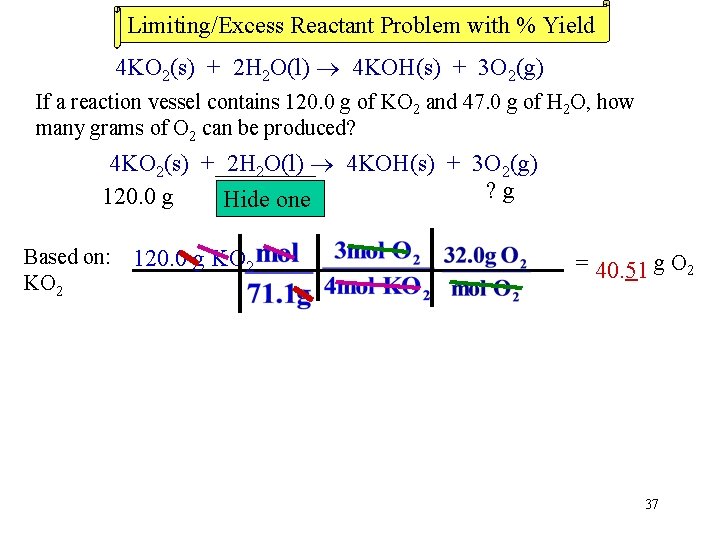
Limiting/Excess Reactant Problem with % Yield 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) If a reaction vessel contains 120. 0 g of KO 2 and 47. 0 g of H 2 O, how many grams of O 2 can be produced? 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) ? g 120. 0 g 47. 0 one g Hide Based on: 120. 0 g KO 2 = 40. 51 g O 2 37

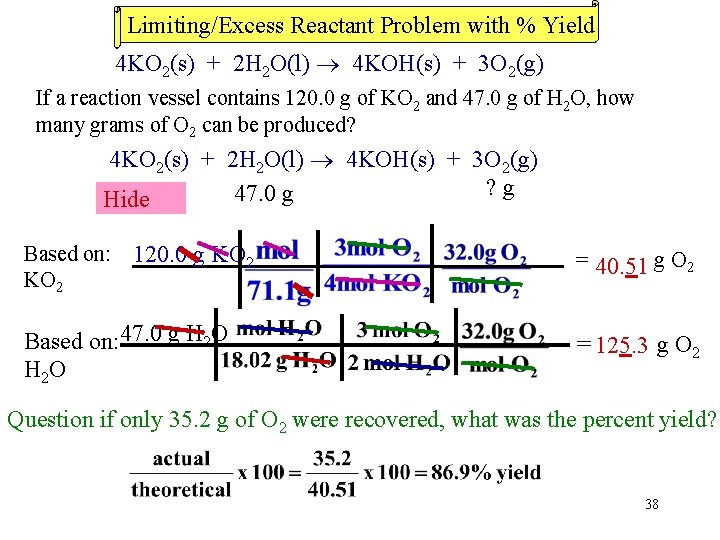
Limiting/Excess Reactant Problem with % Yield 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) If a reaction vessel contains 120. 0 g of KO 2 and 47. 0 g of H 2 O, how many grams of O 2 can be produced? 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) ? g 120. 0 47. 0 g Hide g Based on: 120. 0 g KO 2 = 40. 51 g O 2 Based on: 47. 0 g H 2 O = 125. 3 g O 2 Question if only 35. 2 g of O 2 were recovered, what was the percent yield? 38

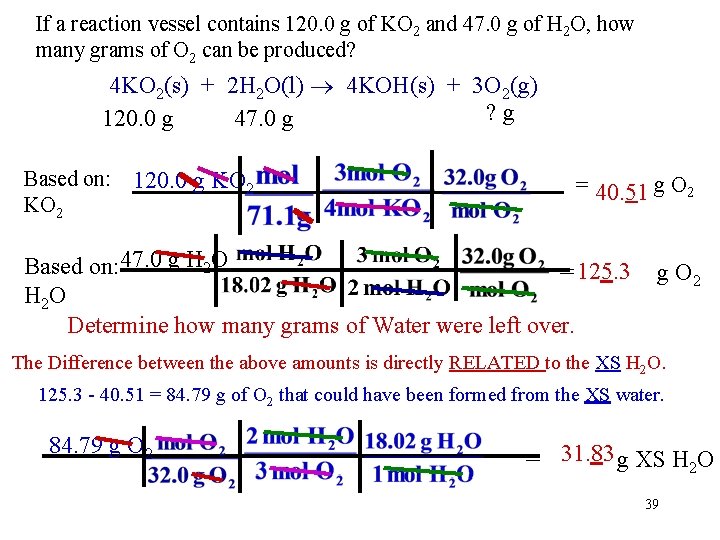
If a reaction vessel contains 120. 0 g of KO 2 and 47. 0 g of H 2 O, how many grams of O 2 can be produced? 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) ? g 120. 0 g 47. 0 g Based on: 120. 0 g KO 2 = 40. 51 g O 2 Based on: 47. 0 g H 2 O = 125. 3 H 2 O Determine how many grams of Water were left over. g O 2 The Difference between the above amounts is directly RELATED to the XS H 2 O. 125. 3 - 40. 51 = 84. 79 g of O 2 that could have been formed from the XS water. 84. 79 g O 2 = 31. 83 g XS H 2 O 39