MOLE CALCULATIONS Moles to Mass n n n




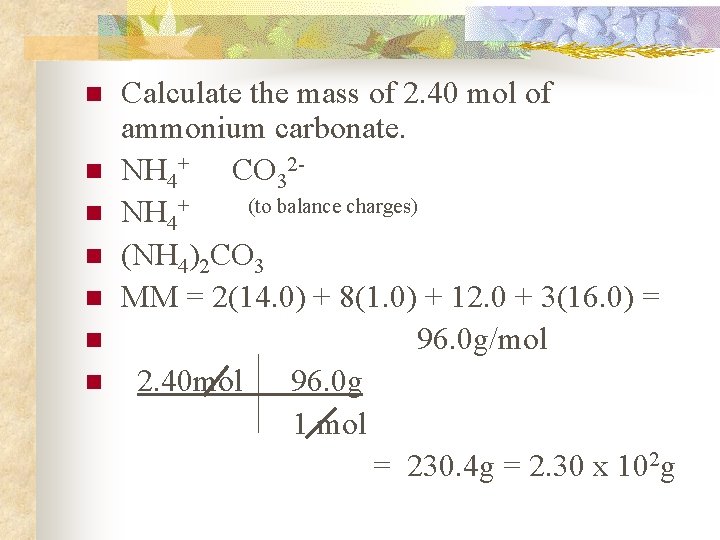
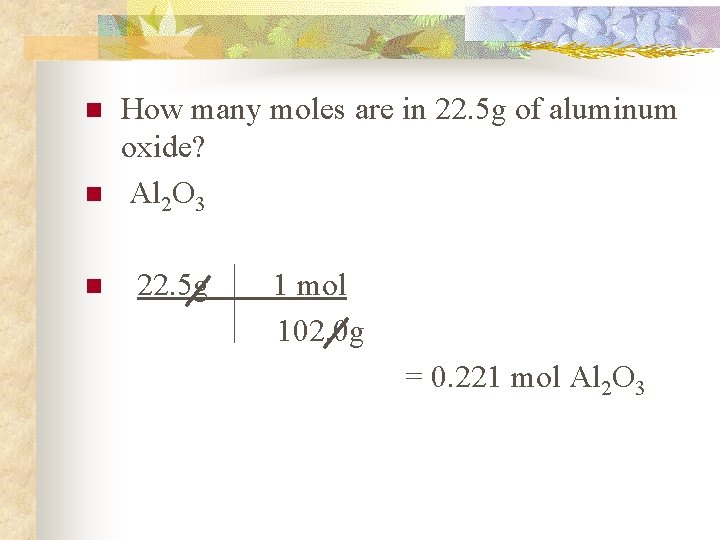







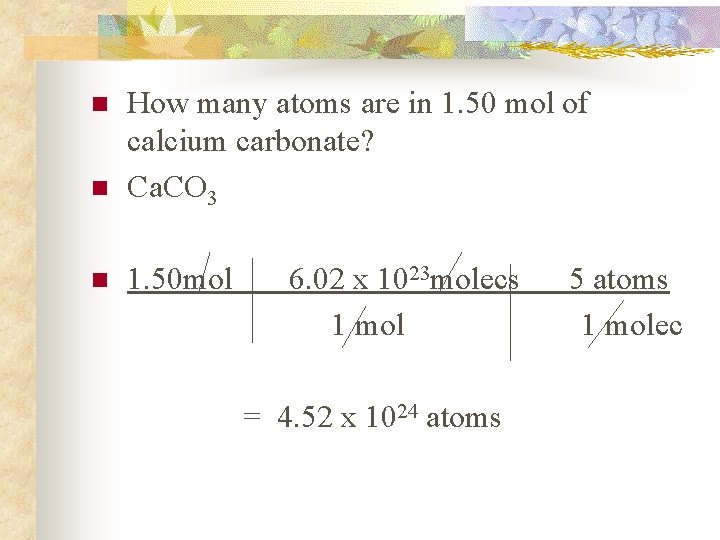

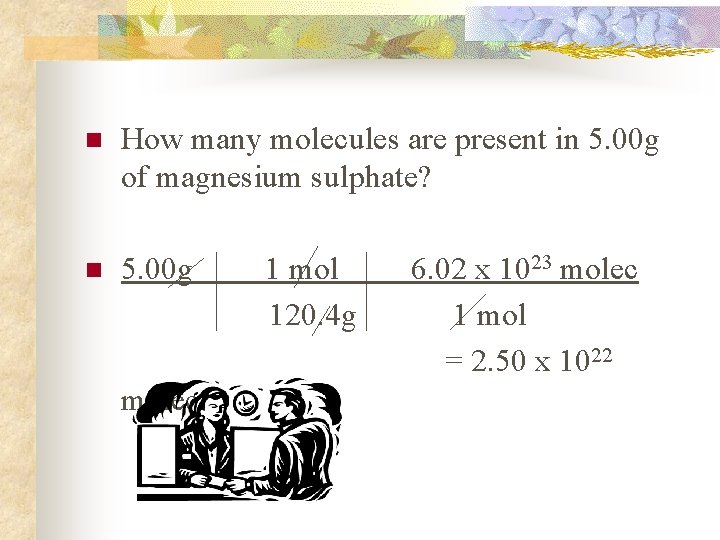



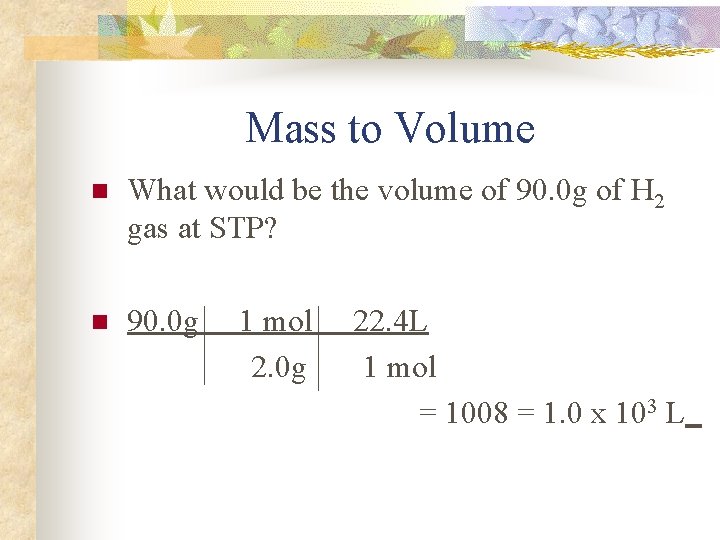

- Slides: 35

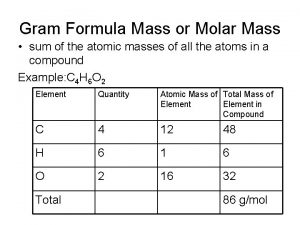
MOLE CALCULATIONS

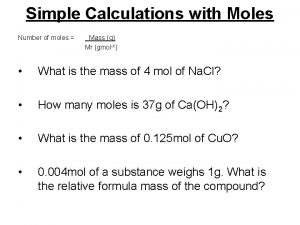
Moles to Mass n n n Find the mass of one mole of Na. Cl 58. 5 g Find the mass of two moles of Na. Cl 117. 0 g How did you get the answer? 2 moles x 58. 5 g/mol = 117. 0 g

n n n What is the mass of 0. 500 moles of Na. Cl? 29. 3 g 0. 500 mol x 58. 5 g/mol = 29. 3 g Find the mass of 0. 35 moles of Na. Cl. 0. 35 mol 58. 5 g 1 mol = 20. 475 g = 2. 0 x 101 g Notice the mole unit cancels (one on top, one on the bottom), leaving only the gram unit!

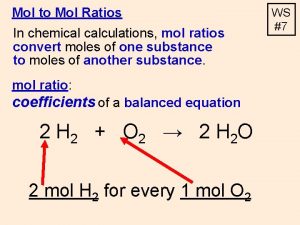
n n Using a table: starting value unit we want for answer unit we have for starting value Top left and bottom right units cancel, left with unit we want in answer (the top right unit)

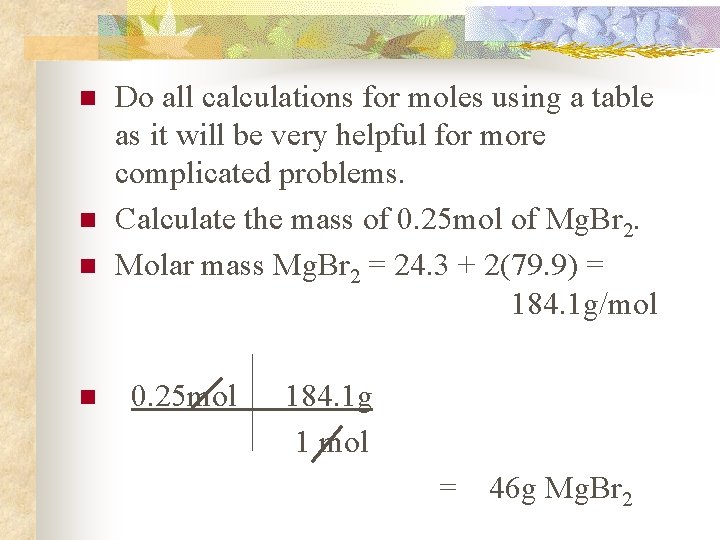
n n Do all calculations for moles using a table as it will be very helpful for more complicated problems. Calculate the mass of 0. 25 mol of Mg. Br 2. Molar mass Mg. Br 2 = 24. 3 + 2(79. 9) = 184. 1 g/mol 0. 25 mol 184. 1 g 1 mol = 46 g Mg. Br 2

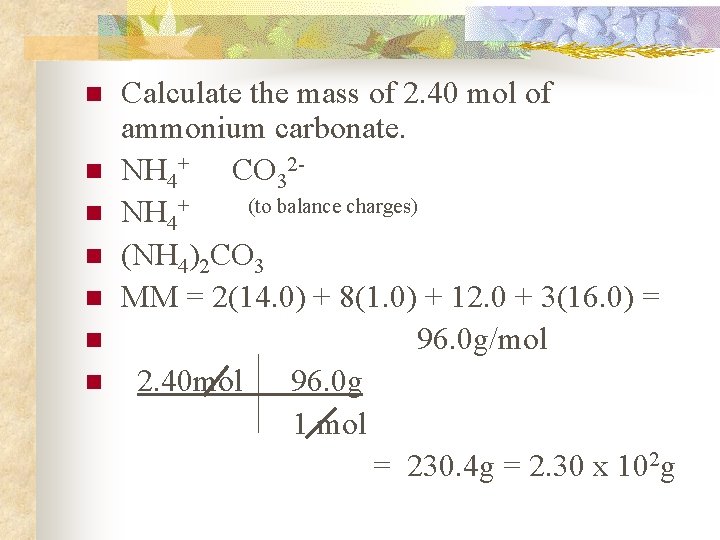
n n n n Calculate the mass of 2. 40 mol of ammonium carbonate. NH 4+ CO 32(to balance charges) NH 4+ (NH 4)2 CO 3 MM = 2(14. 0) + 8(1. 0) + 12. 0 + 3(16. 0) = 96. 0 g/mol 2. 40 mol 96. 0 g 1 mol = 230. 4 g = 2. 30 x 102 g

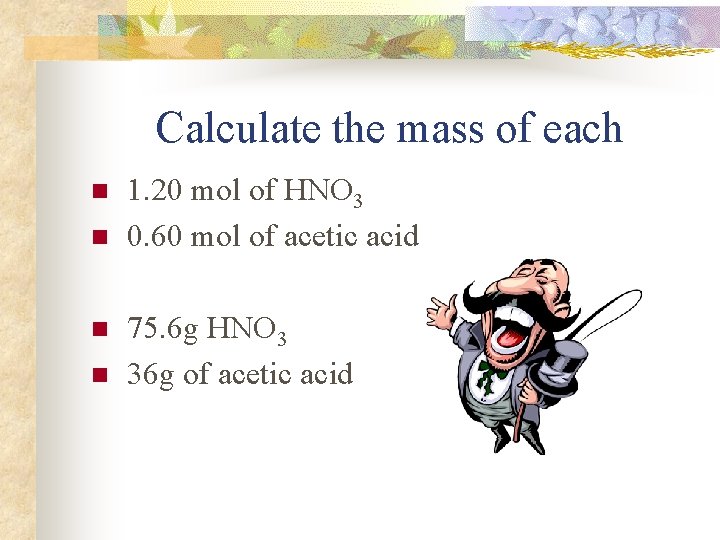
Calculate the mass of each n n 1. 20 mol of HNO 3 0. 60 mol of acetic acid 75. 6 g HNO 3 36 g of acetic acid

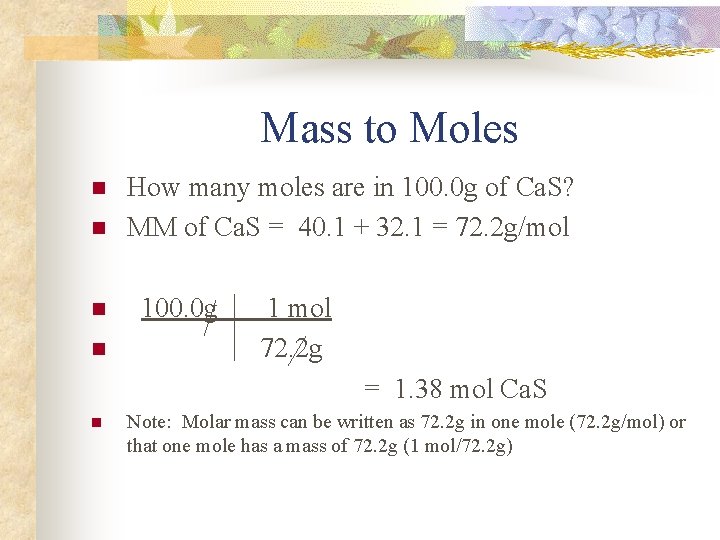
Mass to Moles n n How many moles are in 100. 0 g of Ca. S? MM of Ca. S = 40. 1 + 32. 1 = 72. 2 g/mol 100. 0 g 1 mol 72. 2 g = 1. 38 mol Ca. S n Note: Molar mass can be written as 72. 2 g in one mole (72. 2 g/mol) or that one mole has a mass of 72. 2 g (1 mol/72. 2 g)
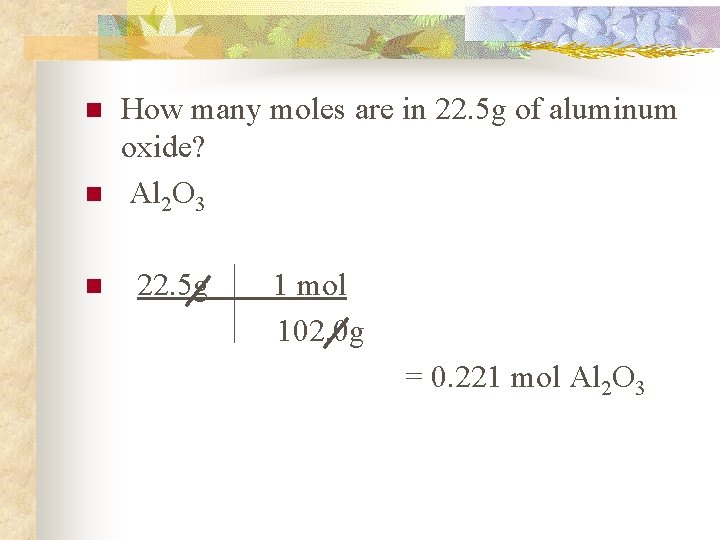
n n n How many moles are in 22. 5 g of aluminum oxide? Al 2 O 3 22. 5 g 1 mol 102. 0 g = 0. 221 mol Al 2 O 3

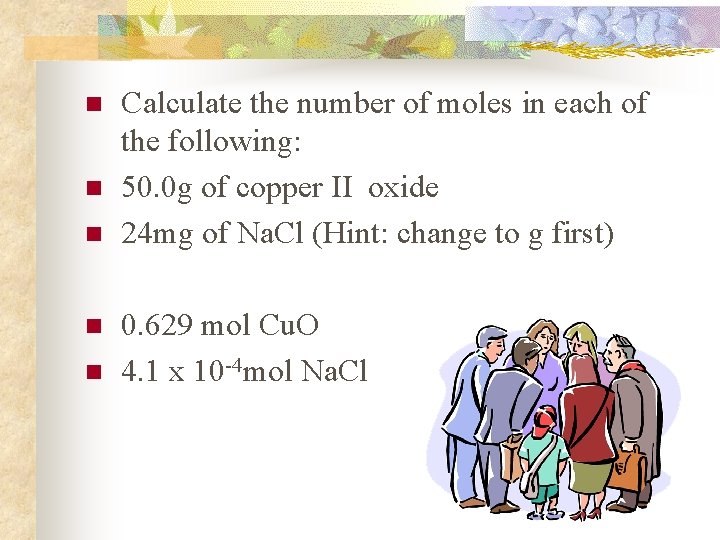
n n n Calculate the number of moles in each of the following: 50. 0 g of copper II oxide 24 mg of Na. Cl (Hint: change to g first) 0. 629 mol Cu. O 4. 1 x 10 -4 mol Na. Cl


Moles to Molecules n n n We must use Avogadro’s number There are 6. 02 x 1023 particles in 1 mole How many molecules are in 1. 00 mole of Na. Cl? 6. 02 x 1023 molecules How many molecules are in 2. 00 mole of Na. Cl? 1. 20 x 1024 molecules

n n n How many molecules are in 0. 500 mole of Na. Cl? 3. 01 x 1023 molecules How many molecules are in 0. 500 mole of Cu. S? 3. 01 x 1023 molecules Keep in mind that Avogadro’s number is independent of what type of molecule or object we are talking about. Mass is dependent because every type of atom has a different mass!

n n n Use the TABLE for all questions! How many molecules are in 1. 20 mol of Pb. SO 4? 1. 20 mol 6. 02 x 1023 molecs 1 mol = 7. 22 x 1023 molecs

n n How many molecules in 1. 30 mol of potassium chloride? 1. 30 mol 6. 02 x 1023 molecs 1 mol = 7. 83 x 1023 molecs

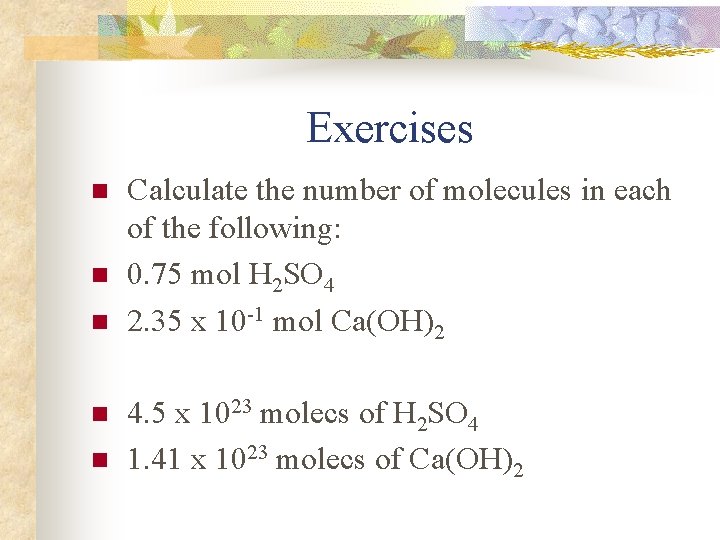
Exercises n n n Calculate the number of molecules in each of the following: 0. 75 mol H 2 SO 4 2. 35 x 10 -1 mol Ca(OH)2 4. 5 x 1023 molecs of H 2 SO 4 1. 41 x 1023 molecs of Ca(OH)2

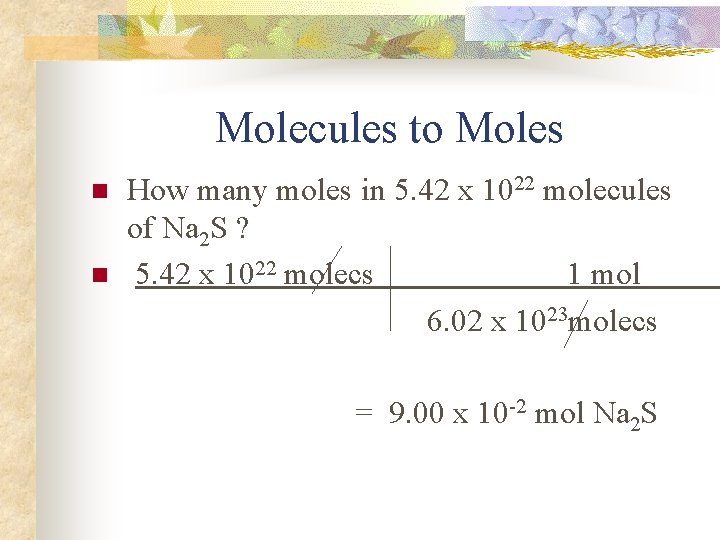
Molecules to Moles n n How many moles in 5. 42 x 1022 molecules of Na 2 S ? 5. 42 x 1022 molecs 1 mol 6. 02 x 1023 molecs = 9. 00 x 10 -2 mol Na 2 S

Exercise n How many moles in 3. 21 x 1023 molecules of KBr ? n 0. 533 mol KBr

Moles to Atoms n n If we are dealing with an element, do moles to atoms just as you did moles to molecules If we are dealing with compounds, we must use a two step table

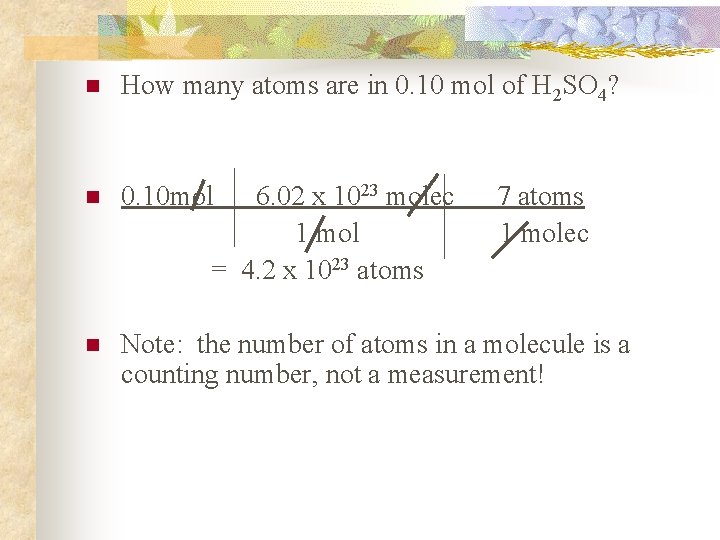
n How many atoms are in 0. 10 mol of H 2 SO 4? n 0. 10 mol n Note: the number of atoms in a molecule is a counting number, not a measurement! 6. 02 x 1023 molec 1 mol = 4. 2 x 1023 atoms 7 atoms 1 molec
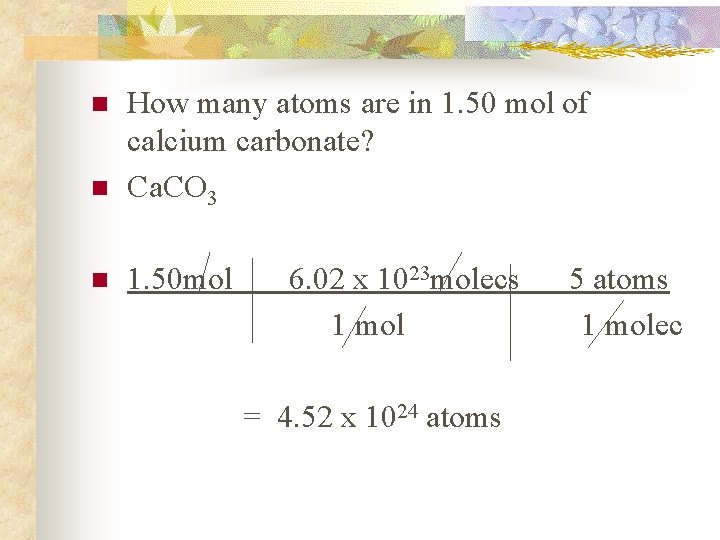
n How many atoms are in 1. 50 mol of calcium carbonate? Ca. CO 3 n 1. 50 mol n 6. 02 x 1023 molecs 1 mol = 4. 52 x 1024 atoms 5 atoms 1 molec

Exercise n Determine the number of atoms in 0. 65 mol Mg(OH)2. n 2. 0 x 1024 atoms

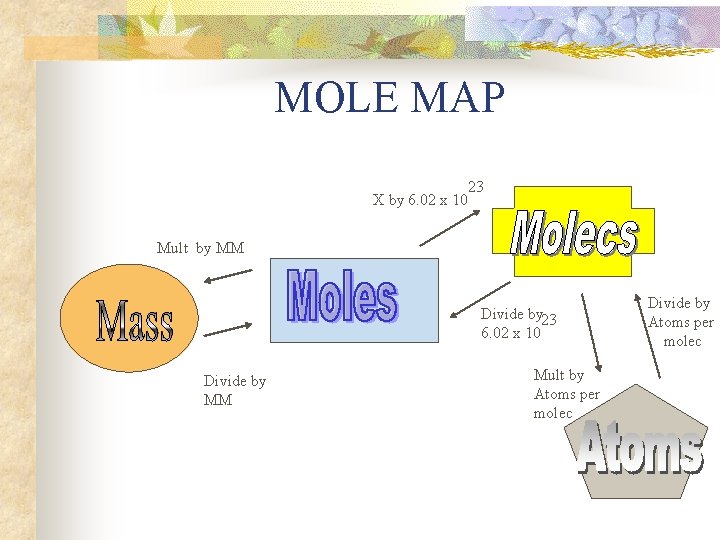
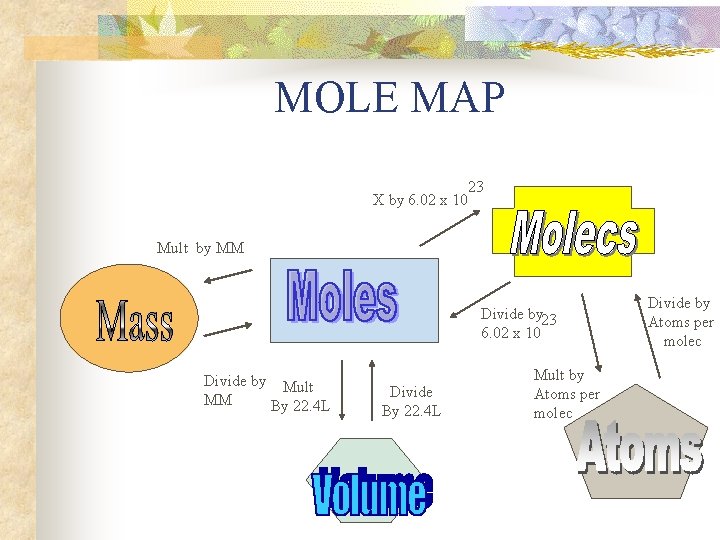
MOLE MAP X by 6. 02 x 10 23 Mult by MM Divide by 23 6. 02 x 10 Divide by MM Mult by Atoms per molec Divide by Atoms per molec

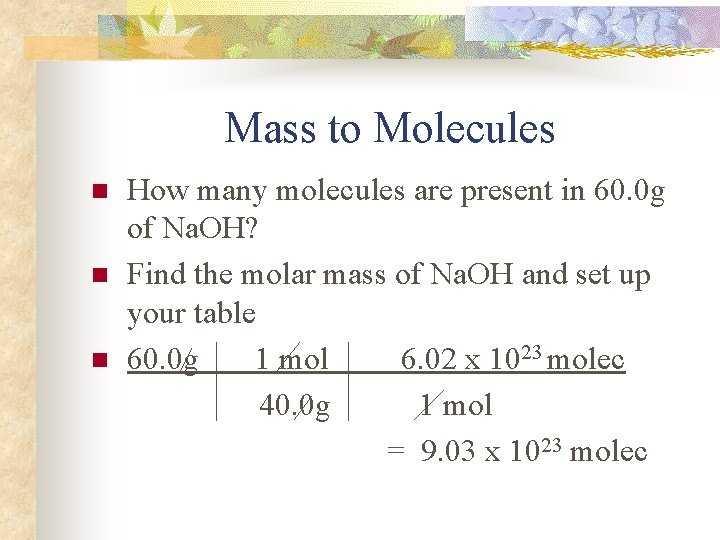
Mass to Molecules n n n How many molecules are present in 60. 0 g of Na. OH? Find the molar mass of Na. OH and set up your table 60. 0 g 1 mol 6. 02 x 1023 molec 40. 0 g 1 mol = 9. 03 x 1023 molec
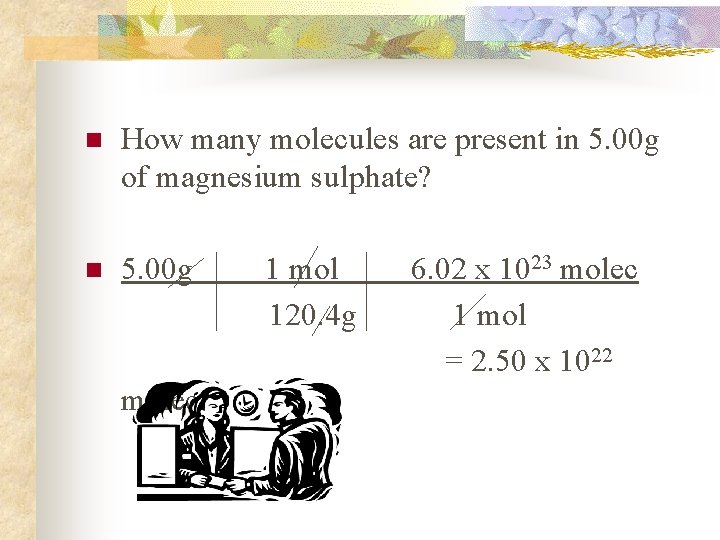
n How many molecules are present in 5. 00 g of magnesium sulphate? n 5. 00 g molec 1 mol 120. 4 g 6. 02 x 1023 molec 1 mol = 2. 50 x 1022

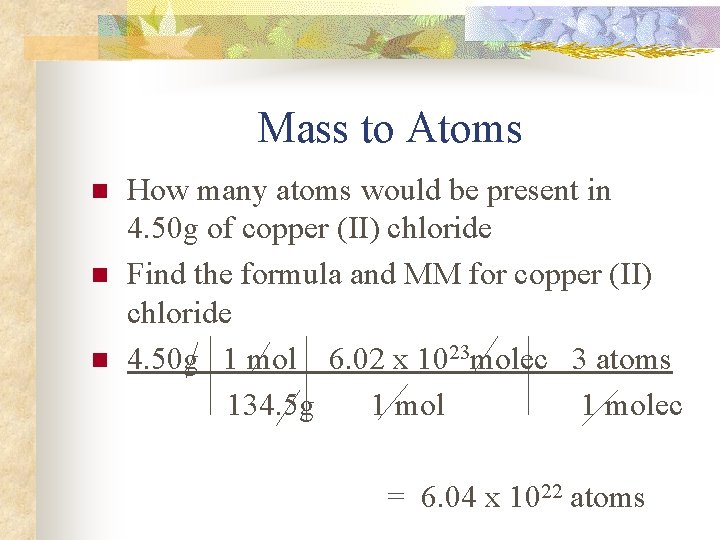
Mass to Atoms n n n How many atoms would be present in 4. 50 g of copper (II) chloride Find the formula and MM for copper (II) chloride 4. 50 g 1 mol 6. 02 x 1023 molec 3 atoms 134. 5 g 1 molec = 6. 04 x 1022 atoms

Exercise n n How many carbon atoms would be present in 795. 0 mg of acetic acid? 0. 795 g 1 mol 6. 02 x 1023 molec 2 atoms 60. 0 g 1 molec = 1. 60 x 1022 carbon atoms


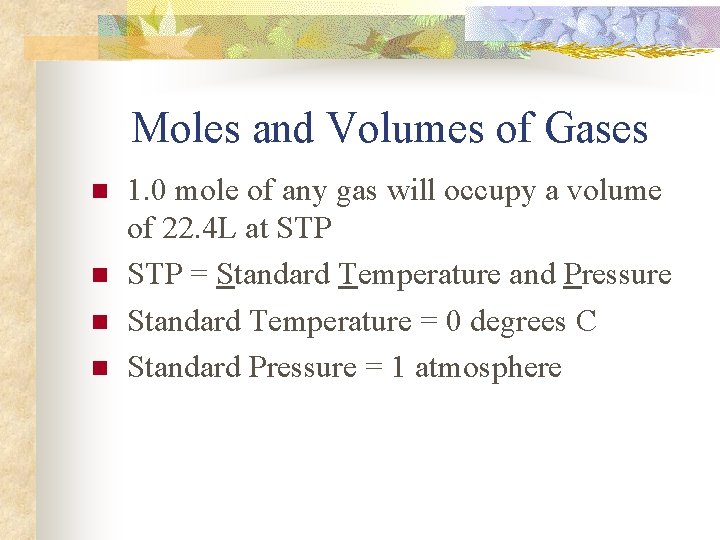
Moles and Volumes of Gases n n 1. 0 mole of any gas will occupy a volume of 22. 4 L at STP = Standard Temperature and Pressure Standard Temperature = 0 degrees C Standard Pressure = 1 atmosphere

Moles to Volume n n How much space does 0. 563 moles of H 2 gas take up at STP? 0. 563 mol 22. 4 L 1 mol = 12. 6 L

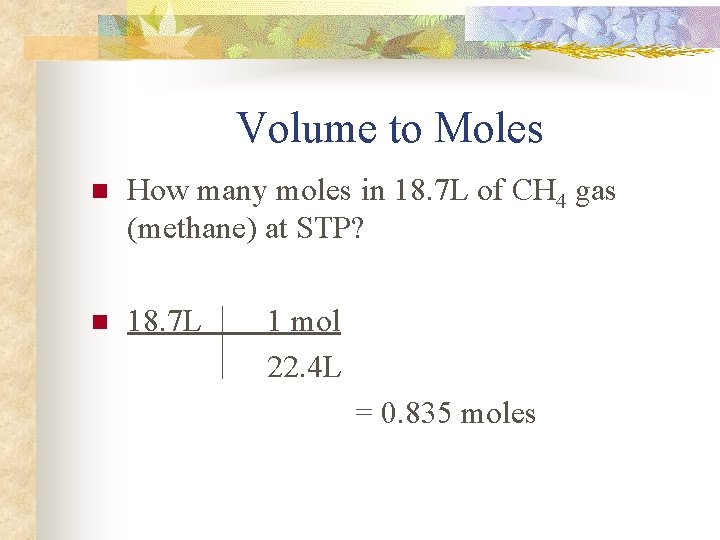
Volume to Moles n How many moles in 18. 7 L of CH 4 gas (methane) at STP? n 18. 7 L 1 mol 22. 4 L = 0. 835 moles
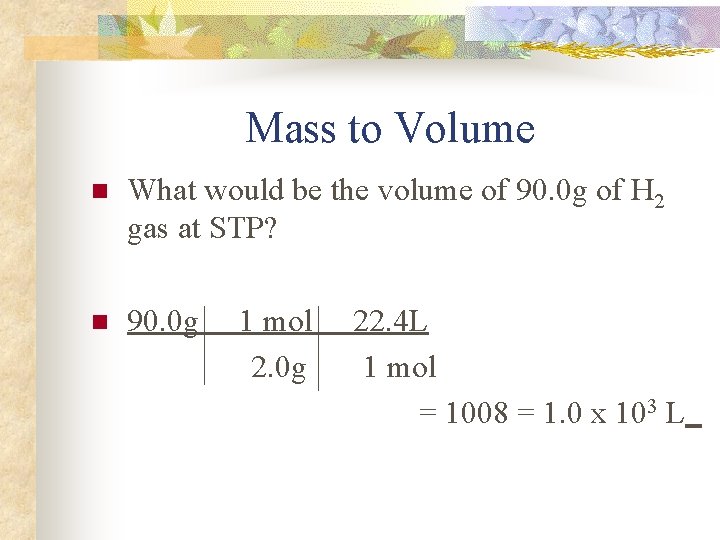
Mass to Volume n What would be the volume of 90. 0 g of H 2 gas at STP? n 90. 0 g 1 mol 2. 0 g 22. 4 L 1 mol = 1008 = 1. 0 x 103 L

MOLE MAP X by 6. 02 x 10 23 Mult by MM Divide by 23 6. 02 x 10 Divide by Mult MM By 22. 4 L Divide By 22. 4 L Mult by Atoms per molec Divide by Atoms per molec

 Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Mole mass and mole volume relationships
Mole mass and mole volume relationships Types of connections in steel structures
Types of connections in steel structures Ideal gas law powerpoint
Ideal gas law powerpoint Mole calculations
Mole calculations Xkcd mole of moles
Xkcd mole of moles Mole-mole factor
Mole-mole factor Molar mass of sucrose
Molar mass of sucrose Stoichiometry mole-mole
Stoichiometry mole-mole Stoichiometry mole-mole problems
Stoichiometry mole-mole problems The mole bridge
The mole bridge How to convert mg to moles
How to convert mg to moles Moles mass rfm triangle
Moles mass rfm triangle Convert from mass to moles
Convert from mass to moles Mol=mass/mr
Mol=mass/mr Alright. how do we then get from moles (n) to mass (m)
Alright. how do we then get from moles (n) to mass (m) Mass gfm triangle
Mass gfm triangle What formula relates moles, mass and mr?
What formula relates moles, mass and mr? How to convert grams to mole
How to convert grams to mole The formula of mole
The formula of mole Mass = moles x mr
Mass = moles x mr Mole by volume
Mole by volume Empirical formula poem
Empirical formula poem Mountains into molehills mass-mole conversions answers
Mountains into molehills mass-mole conversions answers Formula mass vs gram formula mass
Formula mass vs gram formula mass One mole of pennies
One mole of pennies Gravitational mass vs inertial mass
Gravitational mass vs inertial mass Mass to mass formula
Mass to mass formula Mass number formula
Mass number formula Unit for molar mass
Unit for molar mass Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same A rocket of mass 12000 kg accelerates vertically
A rocket of mass 12000 kg accelerates vertically Molar mass table
Molar mass table Inertial mass vs gravitational mass
Inertial mass vs gravitational mass Isotopes
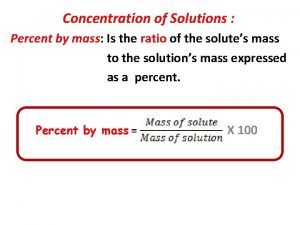
Isotopes How to find percent concentration
How to find percent concentration