THE MOLE CONTENTS What is a mole and













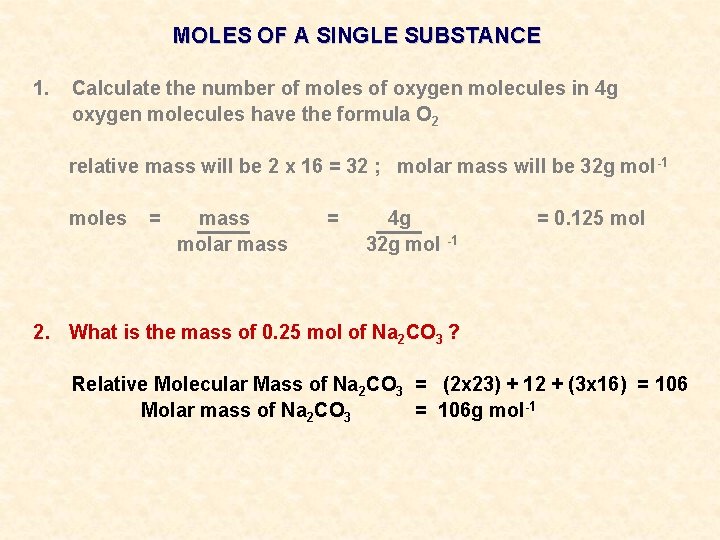
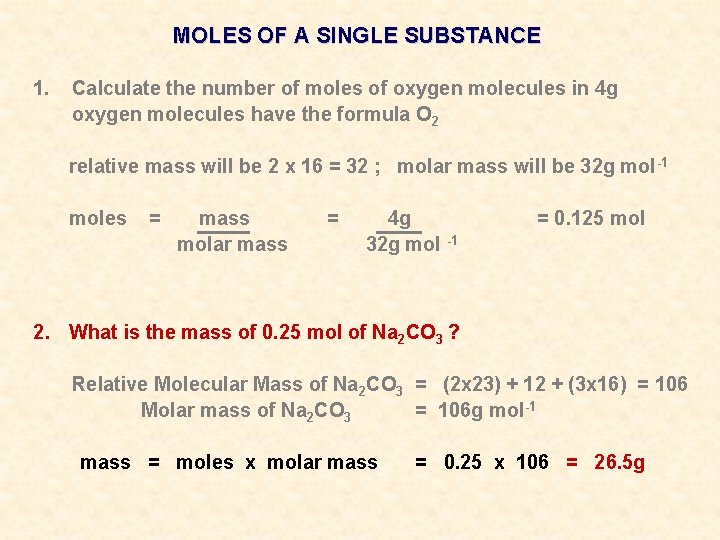
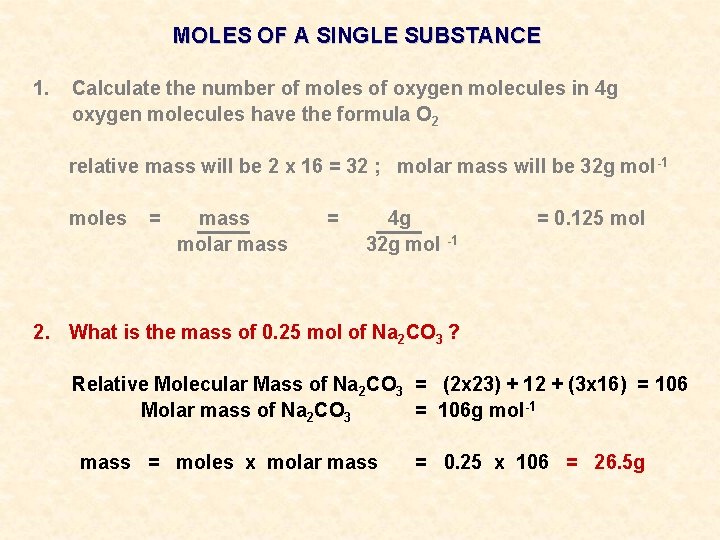
- Slides: 18

THE MOLE CONTENTS • What is a mole and why do we use it? • Calculating the number of moles of a single substance • Reacting mass calculations

THE MOLE Before you start it would be helpful to… • know how to balance simple equations • know how to re-arrange mathematical formulae DON’T BE LEFT IN THE DARK!

THE MOLE WHAT IS A MOLE ? it is the standard unit of amount of a substance it is just a number, a very big number it is a way of saying a number in words, just like. . . DOZEN for 12 SCORE for 20 GROSS for 144

THE MOLE WHAT IS A MOLE ? it is the standard unit of amount of a substance it is just a number, a very big number it is a way of saying a number in words, just like. . . DOZEN for 12 SCORE for 20 GROSS for 144 HOW BIG IS IT ? 60220000000000 (Approximately). . . THAT’S It is a lot easier to write it as. . . BIG !!! 6. 022 x 1023

THE MOLE WHAT IS A MOLE ? it is the standard unit of amount of a substance it is just a number, a very big number it is a way of saying a number in words, just like. . . DOZEN for 12 SCORE for 20 GROSS for 144 HOW BIG IS IT ? 60220000000000 (Approximately). . . THAT’S It is a lot easier to write it as. . . It is also known as. . . BIG !!! 6. 022 x 1023 AVOGADRO’S NUMBER It doesn’t matter what the number is as long as everybody sticks to the same value !

THE MOLE WHY USE IT ? Atoms and molecules don’t weigh much so it is easier to count large numbers of them. In fact it is easier to weigh substances. Using moles tells you. . . how many particles you get in a certain mass the mass of a certain number of particles DO I NEED TO KNOW ANYTHING ELSE ? Yes, it would help if you can balance equations AND Keep trying, you will get the idea. . . EVENTUALLY!

THE MOLE – AN OVERVIEW WHAT IS IT? The standard unit of amount of a substance just as the standard unit of length is a METRE It is just a number, a very big number It is also a way of saying a number in words like DOZEN for 12 GROSS for 144

THE MOLE – AN OVERVIEW WHAT IS IT? The standard unit of amount of a substance just as the standard unit of length is a METRE It is just a number, a very big number It is also a way of saying a number in words like DOZEN for 12 GROSS for 144 HOW BIG IS IT ? 60220000000000 (approx) - THAT’S BIG !!! It is a lot easier to write it as 6. 022 x 1023 And anyway it doesn’t matter what the number is as long as everybody sticks to the same value !

THE MOLE – AN OVERVIEW WHAT IS IT? The standard unit of amount of a substance just as the standard unit of length is a METRE It is just a number, a very big number It is also a way of saying a number in words like DOZEN for 12 GROSS for 144 HOW BIG IS IT ? 60220000000000 (approx) - THAT’S BIG !!! It is a lot easier to write it as 6. 022 x 1023 And anyway it doesn’t matter what the number is as long as everybody sticks to the same value ! WHY USE IT ? Atoms and molecules don’t weigh much so it is easier to count large numbers of them. In fact it is easier to weigh substances. Using moles tells you : - how many particles you get in a certain mass the mass of a certain number of particles

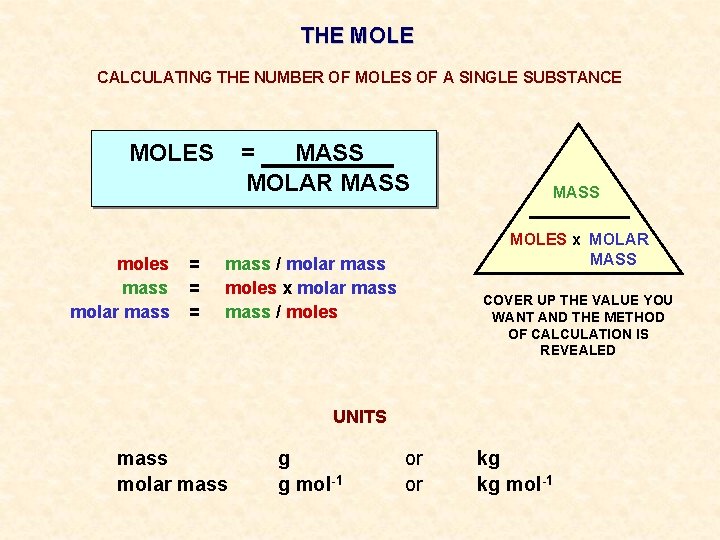
THE MOLE CALCULATING THE NUMBER OF MOLES OF A SINGLE SUBSTANCE MOLES moles mass molar mass = = MASS MOLAR MASS MOLES x MOLAR MASS mass / molar mass moles x molar mass / moles COVER UP THE VALUE YOU WANT AND THE METHOD OF CALCULATION IS REVEALED UNITS mass molar mass g g mol-1 or or kg kg mol-1

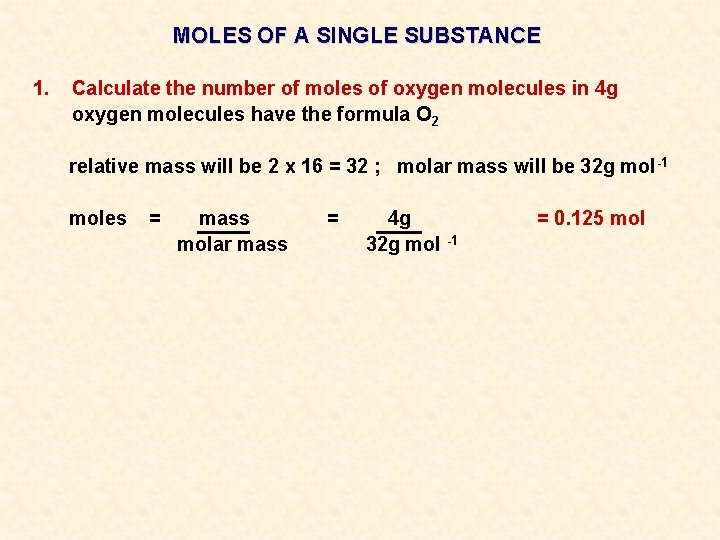
MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g

MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1

MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol

MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol

MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 2. What is the mass of 0. 25 mol of Na 2 CO 3 ? = 0. 125 mol
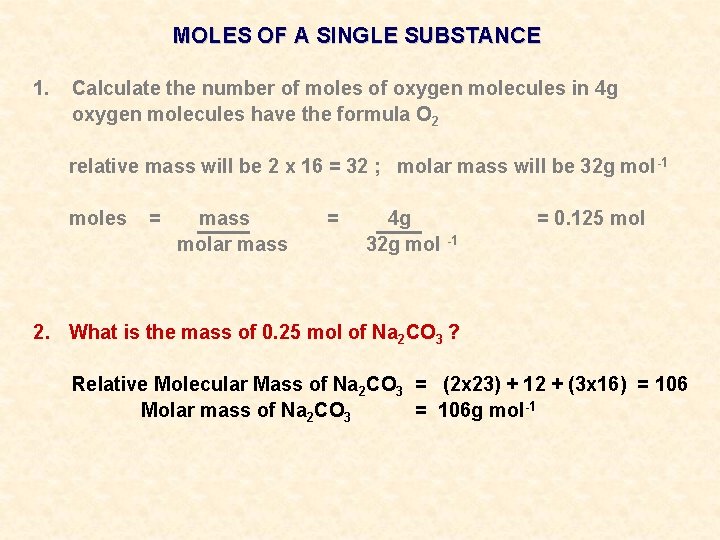
MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol 2. What is the mass of 0. 25 mol of Na 2 CO 3 ? Relative Molecular Mass of Na 2 CO 3 = (2 x 23) + 12 + (3 x 16) = 106 Molar mass of Na 2 CO 3 = 106 g mol-1
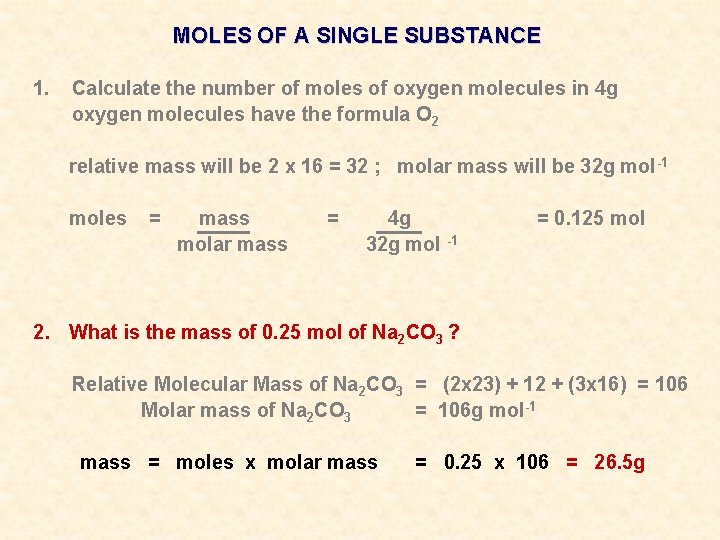
MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol 2. What is the mass of 0. 25 mol of Na 2 CO 3 ? Relative Molecular Mass of Na 2 CO 3 = (2 x 23) + 12 + (3 x 16) = 106 Molar mass of Na 2 CO 3 = 106 g mol-1 mass = moles x molar mass = 0. 25 x 106 = 26. 5 g
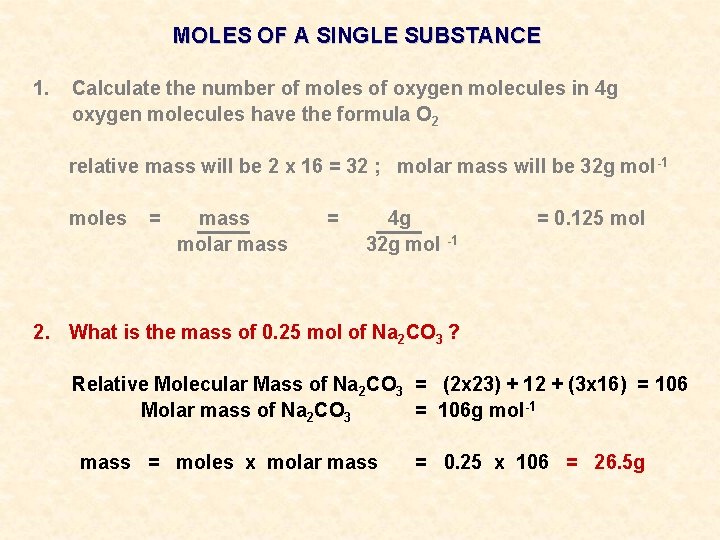
MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol 2. What is the mass of 0. 25 mol of Na 2 CO 3 ? Relative Molecular Mass of Na 2 CO 3 = (2 x 23) + 12 + (3 x 16) = 106 Molar mass of Na 2 CO 3 = 106 g mol-1 mass = moles x molar mass = 0. 25 x 106 = 26. 5 g
 Mole-mass-volume relationships
Mole-mass-volume relationships Mole problem
Mole problem Mole-mole factor
Mole-mole factor Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Phosphorus + oxygen
Phosphorus + oxygen Stoichiometry mole-mole
Stoichiometry mole-mole Molar to grams
Molar to grams Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Glasgow thang điểm
Glasgow thang điểm Chúa yêu trần thế
Chúa yêu trần thế Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ