The MOLE Mole The mole is a word


















- Slides: 22

The MOLE

? Mole The mole is a word that means a very specific number. We know about the word “dozen” it means 12 items. The mole means 6. 0221367 x 1023 particles. we just round it to 6. 022 x 1023.

If we have a mole of anything we have 6. 022 x 1023 of them. That’s a lot of anything to have, unless you think about atoms and molecules. 1 mole of particles = 602, 213, 670, 000, 000 particles The number has made me crazy.

Avogadro’s number Avogadro's number and is denoted by NA. It is derived from exactly 12 grams of carbon-12. 12 grams of carbon -12 has approximately 6. 02 x 1023 atoms. Amadeo Avogadro (1776 -1856)

What do we do with it? It happens to be the SI unit of amount of anything. We use it in chemistry when working with chemical equations. It can stand for: atoms, molecules, or formula units.

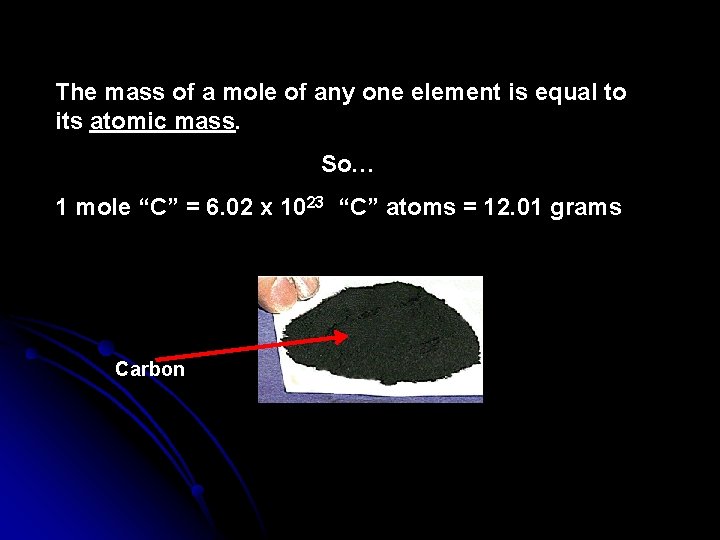
The mass of a mole of any one element is equal to its atomic mass. So… 1 mole “C” = 6. 02 x 1023 “C” atoms = 12. 01 grams Carbon

1 mole C 6. 02 x 1023 atoms 12. 01 g All of these can be filled around to make 3 more conversion factors, for a total of 6.

What is the mass of 2. 5 moles of aluminum? We look on the Periodic table to find the atomic mass of Al. This tells us that 1 mole of Al = 26. 98 grams. Time for math: 2. 5 moles Al x 26. 98 g = 67. 45 g of Al 1 mole of Al

The Key For an example lets use gold (Au). [1 mole of Au = 6. 02 x 1023 atoms of Au = 196. 97 grams of Au] These three numbers are all we need to solve any molar equations dealing with gold. Examples: 1. What is the mass of 4. 5 moles of Au? 2. How many atoms of Au will have a mass of 89. 60 g? 3. How many moles of gold do you have if you have 5. 24 x 1026 atoms of gold? 4. What will be the mass of 5. 24 x 1026 atoms of Gold?

What is the mass of 4. 5 moles of Au? 4. 5 mol Au x 196. 97 g = 886. 36 g Au 1 mole of Au = 6. 02 x 1023 atoms of Au = 196. 97 grams of Au

How many atoms of Au will have a mass of 89. 60 g? 89. 60 g Au X 6. 02 x 1023 Au atoms = 2. 738 x 1023 atoms Au 196. 97 Au You are going to need one of these! 1 mole of Au = 6. 02 x 1023 atoms of Au = 196. 97 grams of Au


Molar mass of an ionic compound & Molar mass of a molecular compound

We have reviewed the mole concept for a single element (gram atomic mass or gam). Example: Na: 1 mole = 22. 99 g or we could put it like this 22. 99 g Na/1 mole Na

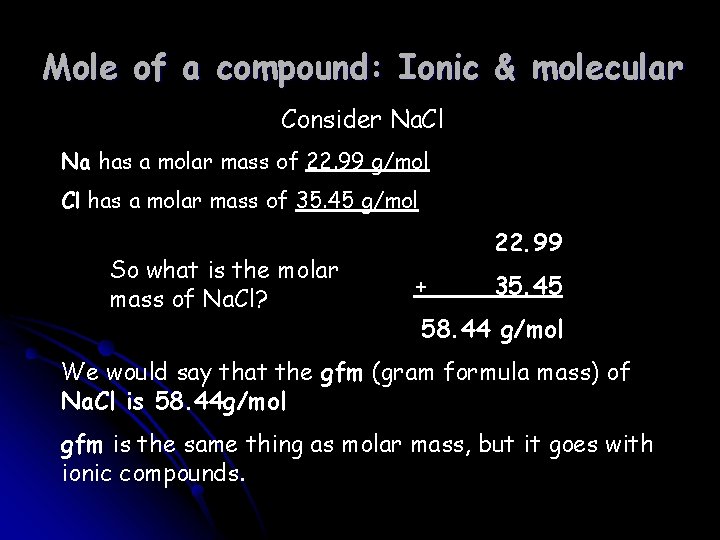
Mole of a compound: Ionic & molecular Consider Na. Cl Na has a molar mass of 22. 99 g/mol Cl has a molar mass of 35. 45 g/mol So what is the molar mass of Na. Cl? 22. 99 + 35. 45 58. 44 g/mol We would say that the gfm (gram formula mass) of Na. Cl is 58. 44 g/mol gfm is the same thing as molar mass, but it goes with ionic compounds.

More examples: One way to determine the molar mass is like this: Fe 2 O 3 Iron (III) oxide Element # of atoms Grams /mol Total mass of that element Fe 2 X 55. 47 = 110. 94 O 3 X 16. 00 = + 48. 00 Total 158. 94 g/mol

More examples: Zn(OH)2 Zn 1 65. 39 “Zinc hydroxide” O 2 16. 00 32. 00 H 2 1. 00 + 2. 00 99. 39 The gfm or molar mass of zinc hydroxide is 99. 39 g/mol

What about molecular compounds Its really the same way! You calculate it. How about water (H 2 O)? Element # of atoms Grams /mol Total mass of that element H 2 X 1. 00 = O 1 X 16. 00 = Total 2. 00 + 16. 00 18. 00 g/mol

Conversion factors 1 mole H 2 O = 18. 00 grams = 6. 02 x 1023 molecules of H 2 O 1 mole H 2 O 18. 00 g H 2 O 18. 00 g H 2 O 1 mole 6. 02 x 1023 molecules Q. What is the mass of 2. 5 moles of water? 2. 5 moles of H 2 O x 18. 00 g H 2 O = 45. 00 g H 2 O 1 mole H 2 O

Mass to moles How many moles of silver nitrate (Ag. NO 3) will have a mass of 55. 0 g? 1 st Ag N O find gfm of Ag. NO 3 1 x 107. 87 = 107. 87 1 x 14. 01 = 14. 01 3 x 16. 00 = + 48. 00 169. 88 g/mol 55. 0 g Ag. NO 3 x 1 mole Ag. NO 3 = 0. 324 mole Ag. NO 3 169. 88 g Ag. NO 3

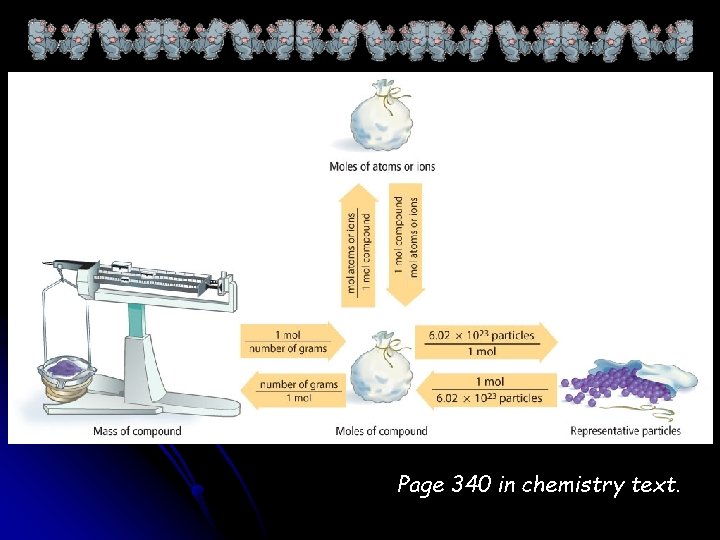
Page 340 in chemistry text.

 Stoichiometry mole-mole problems
Stoichiometry mole-mole problems Mole mole factor
Mole mole factor Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Stoichiometry mole-mole
Stoichiometry mole-mole Stoichiometry mole-mole
Stoichiometry mole-mole Mol si
Mol si Mole mass and mole volume relationships
Mole mass and mole volume relationships Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới