Mole Conversions The Mole n Mole quantity of







- Slides: 11

Mole Conversions

The Mole n Mole – quantity of a substance that has a mass in grams equal to its formula mass. n It is the central unit in converting the amount of a substance from one type of measurement to another.

Mass and Moles: n Use the molar mass or formula mass and calculate the number of moles. n Ex: If you know you have 11. 2 grams of Na. Cl, how many moles of the substance do you have. n 1. Find the formula mass of Na. Cl. 1 X 23 g/mol = 23 g/mol 1 X 35. 5 g/mol = 35. 5 g/mol 58. 5 g/mol

Mass and Moles cont. n 2. Set up your conversion factor. 11. 2 g Na. Cl X 1 mol Na. Cl = 0. 191 mol Na. Cl 58. 5 g Na. Cl Practice: How many grams are in 2. 50 moles of Na. Cl? 146 grams

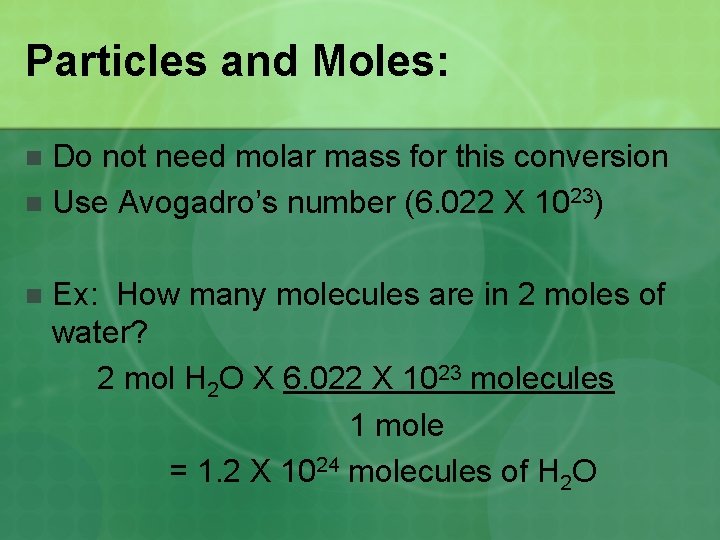
Particles and Moles: Do not need molar mass for this conversion n Use Avogadro’s number (6. 022 X 1023) n n Ex: How many molecules are in 2 moles of water? 2 mol H 2 O X 6. 022 X 1023 molecules 1 mole = 1. 2 X 1024 molecules of H 2 O

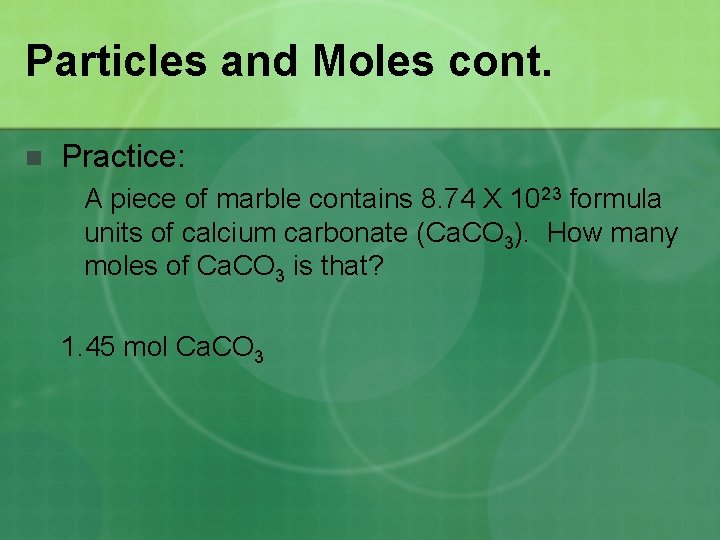
Particles and Moles cont. n Practice: A piece of marble contains 8. 74 X 1023 formula units of calcium carbonate (Ca. CO 3). How many moles of Ca. CO 3 is that? 1. 45 mol Ca. CO 3

Moles and Gases n At the same temperature and pressure, equal volumes of gases contain the same number of gas particles. n 1 mole of any gas at standard temperature and pressure (STP, 0°C and 1 atmosphere) has a volume of 22. 4 liters per mole (22 L/mol).

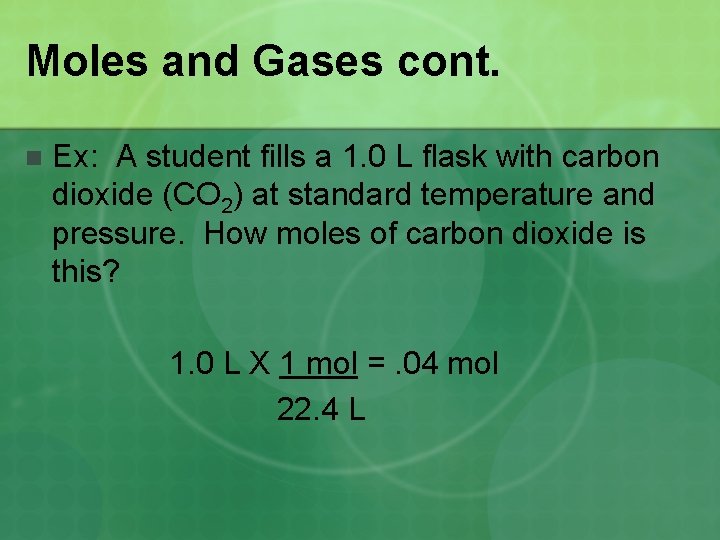
Moles and Gases cont. n Ex: A student fills a 1. 0 L flask with carbon dioxide (CO 2) at standard temperature and pressure. How moles of carbon dioxide is this? 1. 0 L X 1 mol =. 04 mol 22. 4 L

Multistep Conversions: n Always convert to moles and then to the desired amount. n You need 250 grams of table sugar or sucrose (C 12 H 22 O 11), to bake a cake. How many sucrose molecules will be in the cake?

Multistep Conversions cont. 1. Find the formula mass of sucrose. C - 12 X 12 g/mol = 144 g/mol H – 22 X 1. 0 g/mol = 22 g/mol O – 11 X 16 g/mol = 176 g/mol 342 g/mol

Multistep Conversions cont. n 2. Find the moles and then convert to molecules. 250 g X 1 mol =. 73 mol 342 g. 73 mol X 6. 022 X 1023 molecules 1 mol = 4. 4 X 1023 molecules of sucrose