Mole Meme Mole Photos Avogadro Memes MOLE CONVERSIONS

- Slides: 9

Mole Meme & Mole Photos
Avogadro Memes…

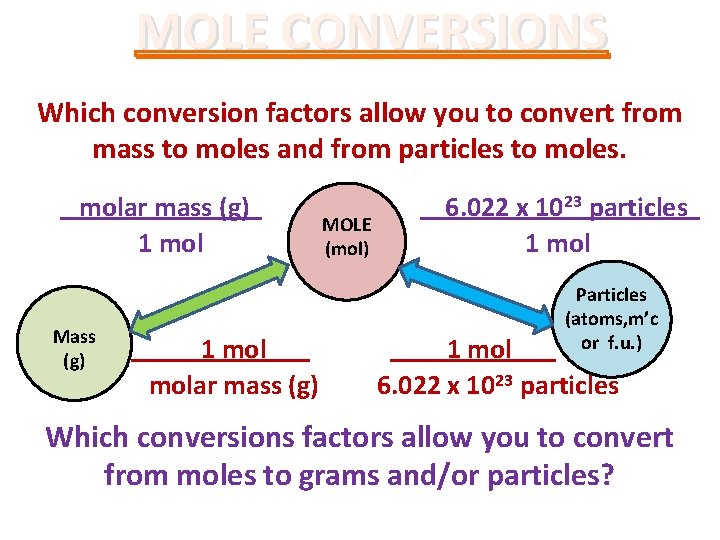
MOLE CONVERSIONS Which conversion factors allow you to convert from mass to moles and from particles to moles. molar mass (g). 1 mol Mass (g) 1 molar mass (g) MOLE (mol) 6. 022 x 1023 particles. . 1 mol Particles (atoms, m’c. or f. u. ) 1 mol 6. 022 x 1023 particles Which conversions factors allow you to convert from moles to grams and/or particles?

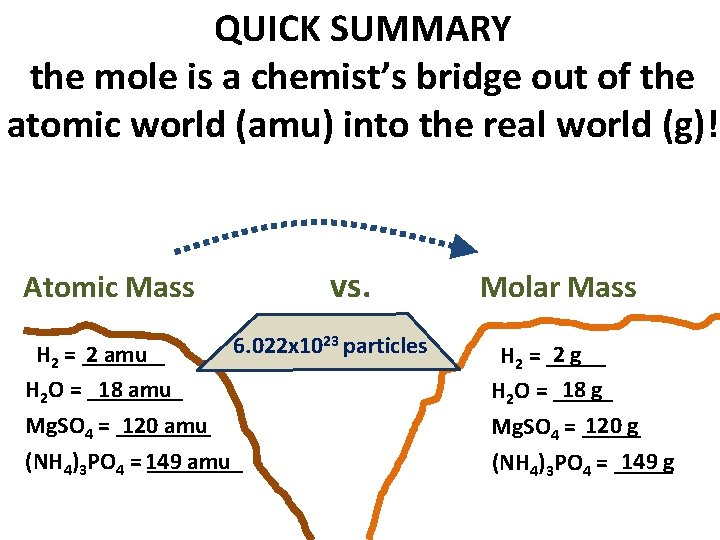
QUICK SUMMARY the mole is a chemist’s bridge out of the atomic world (amu) into the real world (g)! vs. Atomic Mass 6. 022 x 1023 particles H 2 = _______ 2 amu H 2 O = ____ 18 amu Mg. SO 4 = ____ 120 amu (NH 4)3 PO 4 = 149 ____ amu Molar Mass 2 g H 2 = _____ 18 g H 2 O = _____ 120 g Mg. SO 4 = _____ 149 g (NH 4)3 PO 4 = _____

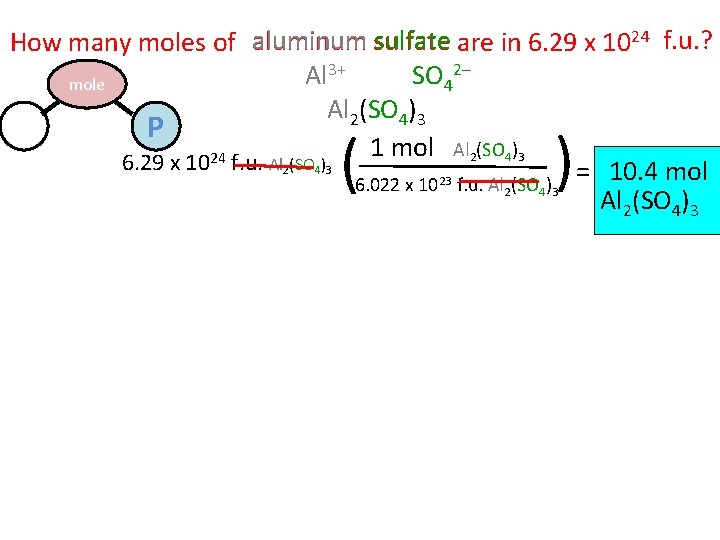
How many moles of aluminum sulfate are in 6. 29 x 1024 f. u. ? 2– 3+ SO Al mole 4 Al 2(SO 4)3 M P 1 mol Al 2(SO 4)3 24 6. 29 x 10 f. u. Al 2(SO 4)3 = 10. 4 mol 6. 022 x 1023 f. u. Al 2(SO 4)3 ( )

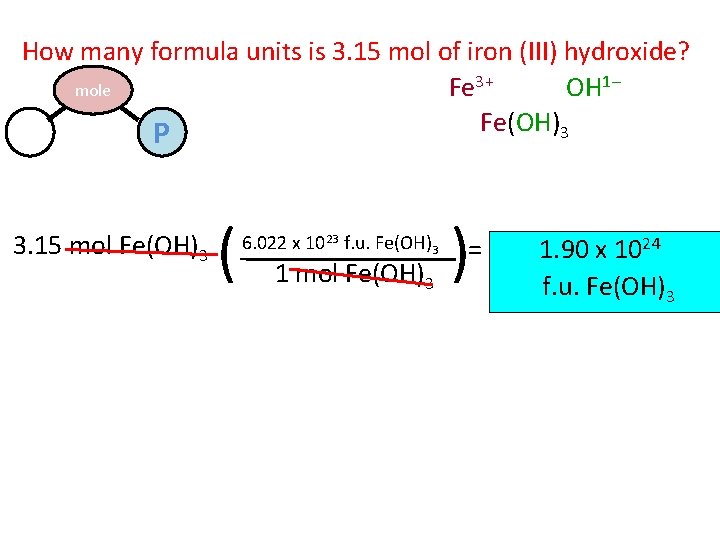
How many formula units is 3. 15 mol of iron (III) hydroxide? Fe 3+ OH 1– mole Fe(OH)3 M P 3. 15 mol Fe(OH)3 ( 6. 022 x 1023 f. u. Fe(OH)3 1 mol Fe(OH)3 ) = 1. 90 x 1024 f. u. Fe(OH)3

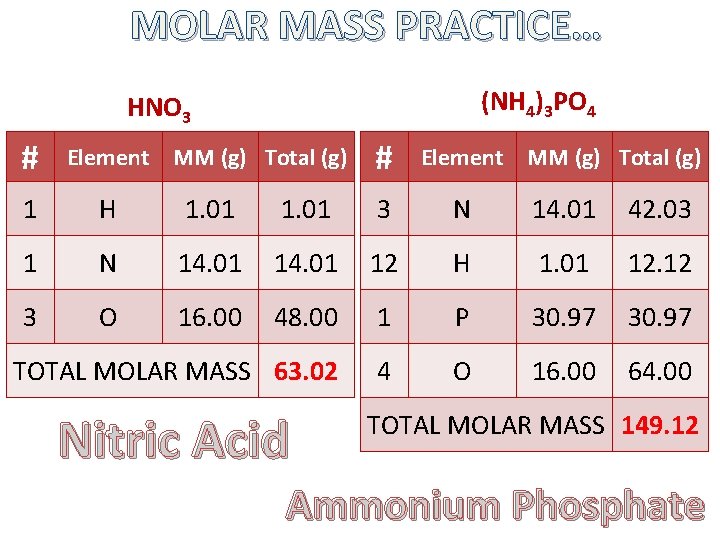
MOLAR MASS PRACTICE… (NH 4)3 PO 4 HNO 3 # Element MM (g) Total (g) 1 H 1. 01 3 N 14. 01 42. 03 1 N 14. 01 12 H 1. 01 12. 12 3 O 16. 00 48. 00 1 P 30. 97 TOTAL MOLAR MASS 63. 02 4 O 16. 00 64. 00 Nitric Acid TOTAL MOLAR MASS 149. 12 Ammonium Phosphate

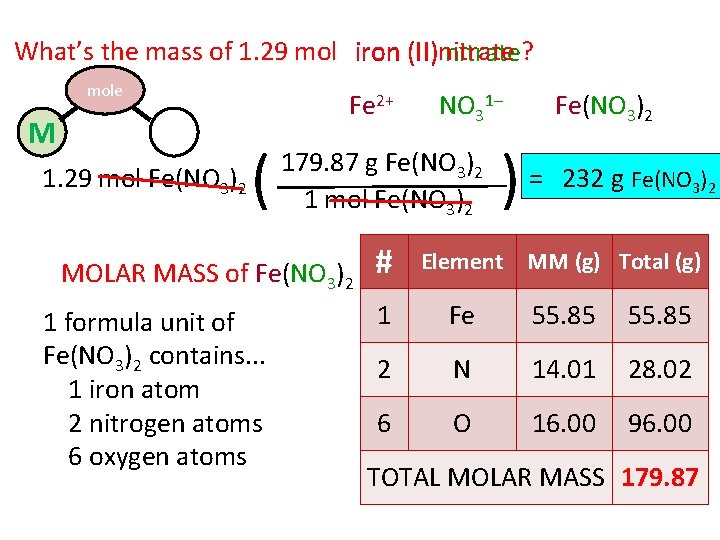
What’s the mass of 1. 29 mol iron (II)nitrate (II) nitrate? mole M P 1. 29 mol Fe(NO 3)2 Fe 2+ ( 179. 87 g Fe(NO 3)2 1 mol Fe(NO 3)2 MOLAR MASS of Fe(NO 3)2 1 formula unit of Fe(NO 3)2 contains. . . 1 iron atom 2 nitrogen atoms 6 oxygen atoms NO 31– # Fe(NO 3)2 ) = 232 g Fe(NO 3)2 Element MM (g) Total (g) 1 Fe 55. 85 2 N 14. 01 28. 02 6 O 16. 00 96. 00 TOTAL MOLAR MASS 179. 87

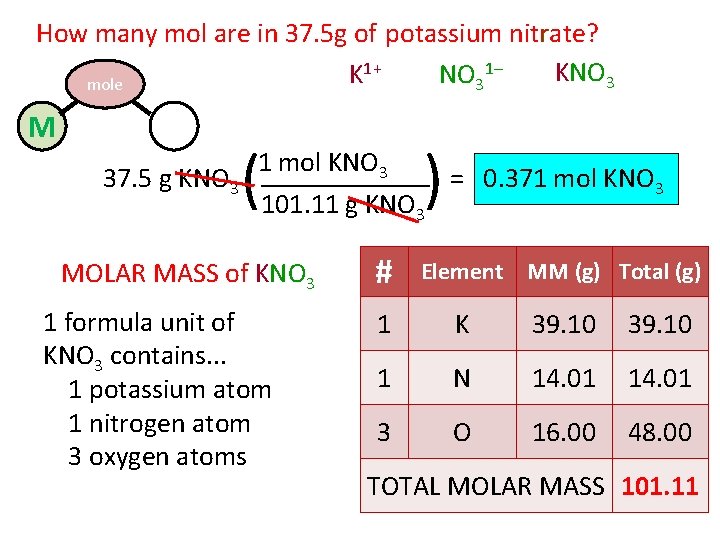
How many mol are in 37. 5 g of potassium nitrate? 1+ 1– KNO 3 K NO 3 mole M P ( ) 1 mol KNO 3 37. 5 g KNO 3 = 0. 371 mol KNO 3 101. 11 g KNO 3 MOLAR MASS of KNO 3 1 formula unit of KNO 3 contains. . . 1 potassium atom 1 nitrogen atom 3 oxygen atoms # Element MM (g) Total (g) 1 K 39. 10 1 N 14. 01 3 O 16. 00 48. 00 TOTAL MOLAR MASS 101. 11