MOLE MEME MOLE PHOTOS AVOGADRO MEMES INTRO TO







- Slides: 14

MOLE MEME & MOLE PHOTOS

AVOGADRO MEMES…

INTRO TO MOLE HTTPS: //WWW. YOUTUBE. COM/WATCH? V=TEL 4 JEETVMG&T= 1 S

THE MOLE… 1. Is a number 6. 022 x 1023 2. Relates number of particles to mass

PARTICLE TYPES • Atoms: element (carbon, oxygen, sodium, etc. ) • Molecules: covalent bonds (water, carbon dioxide, non-metal with non-metal) • Formula Units (f. u. s!): ionic bonds (sodium chloride, hydrochloric acid, non-metal with metal)

CONVERSION PROCEDURE 1. Write out given – Triple Threat! 2. Leave space 3. Write out units and chemical formula of answer 4. Identify conversion factor 5. Write out units of c. f. 6. Write out numbers of c. f. 7. Multiply and divide to get final answer

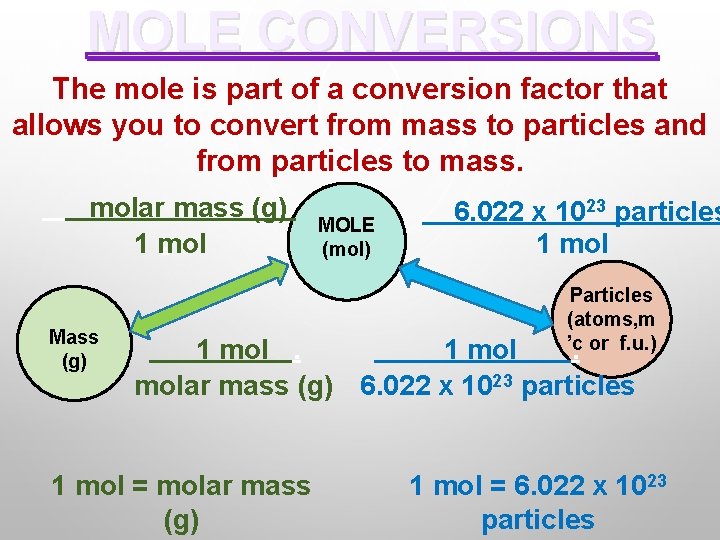
MOLE CONVERSIONS The mole is part of a conversion factor that allows you to convert from mass to particles and from particles to mass. molar mass (g). 1 mol Mass (g) MOLE (mol) 6. 022 x 1023 particles 1 mol Particles (atoms, m ’c. or f. u. ) 1 molar mass (g) 6. 022 x 1023 particles 1 mol = molar mass (g) 1 mol = 6. 022 x 1023 particles

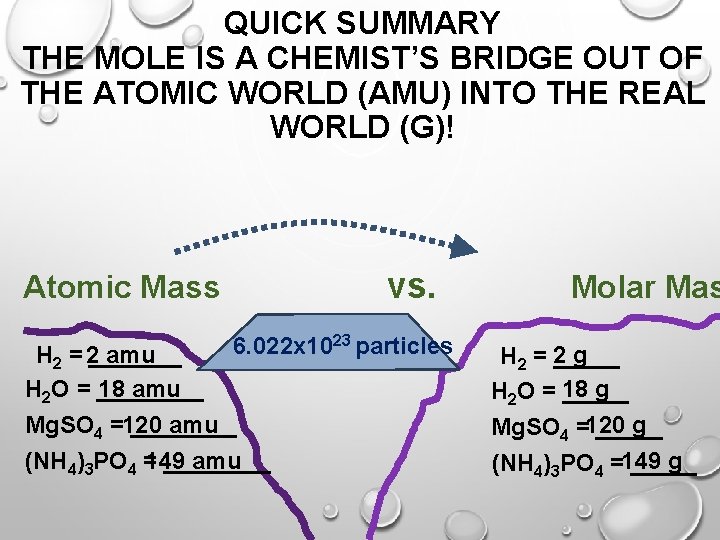
QUICK SUMMARY THE MOLE IS A CHEMIST’S BRIDGE OUT OF THE ATOMIC WORLD (AMU) INTO THE REAL WORLD (G)! vs. Atomic Mass 6. 022 x 1023 particles H 2 = 2_______ amu H 2 O = ____ 18 amu Mg. SO 4 =120 ____ amu (NH 4)3 PO 4 = 149 ____ amu Molar Mas g H 2 = 2 _____ g H 2 O = 18 _____ g Mg. SO 4 =120 _____ g (NH 4)3 PO 4 =149 _____

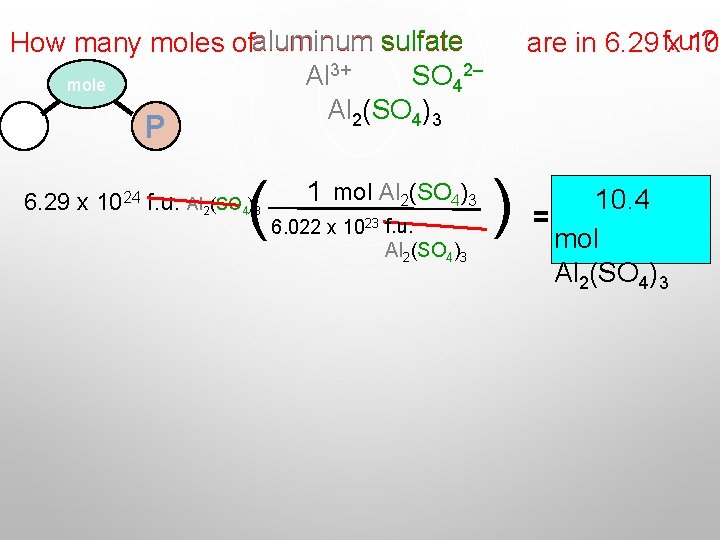
How many moles ofaluminum sulfate SO 42– Al 3+ mole Al 2(SO 4)3 M are in 6. 29 f. u. ? x 10 P ( 6. 29 x 1024 f. u. Al 2(SO 4)3 1 mol Al 2(SO 4)3 6. 022 x 1023 f. u. Al 2(SO 4)3 ) 10. 4 = mol Al 2(SO 4)3

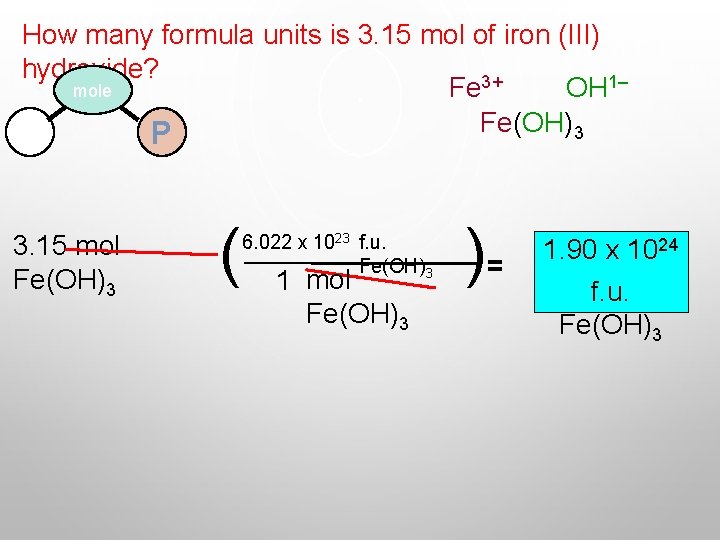
How many formula units is 3. 15 mol of iron (III) hydroxide? mole Fe 3+ OH 1– Fe(OH)3 M P 3. 15 mol Fe(OH)3 ( 6. 022 x 1023 f. u. Fe(OH)3 1 mol Fe(OH)3 )= 1. 90 x 1024 f. u. Fe(OH)3

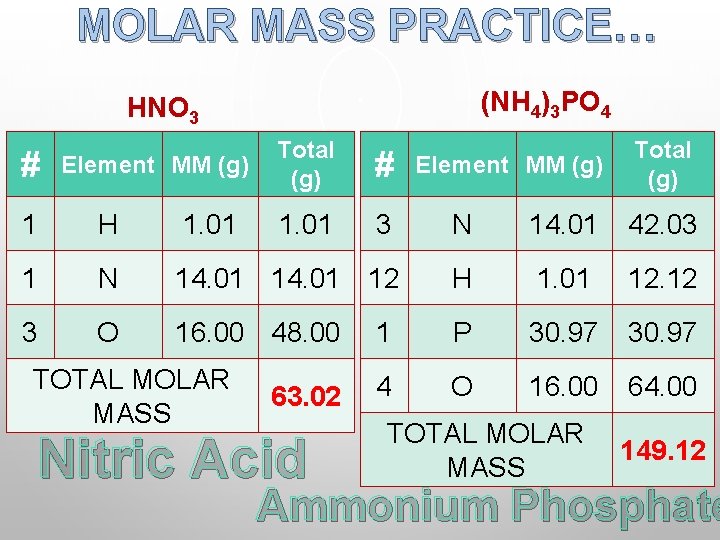
MOLAR MASS PRACTICE… (NH 4)3 PO 4 HNO 3 # Element MM (g) Total (g) # 1. 01 Element MM (g) Total (g) 1 H 1. 01 3 N 14. 01 42. 03 1 N 14. 01 12 H 1. 01 12. 12 3 O 16. 00 48. 00 1 P 30. 97 4 O 16. 00 64. 00 TOTAL MOLAR MASS 63. 02 Nitric Acid TOTAL MOLAR MASS 149. 12 Ammonium Phosphate

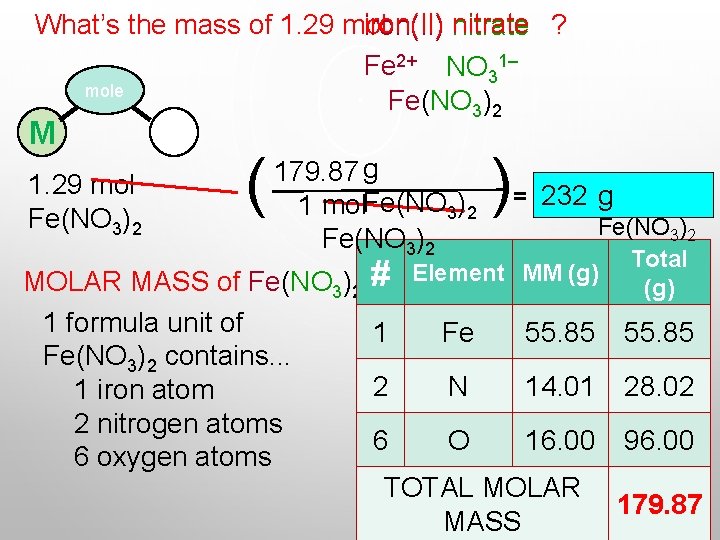
What’s the mass of 1. 29 mol iron(II) nitrate ? Fe 2+ NO 31– mole Fe(NO 3)2 M 1. 29 mol Fe(NO 3)2 P ( 179. 87 g 1 mol. Fe(NO 3)2 ) = 232 g Fe(NO 3)2 Total Element MM (g) MOLAR MASS of Fe(NO 3)2 # 1 formula unit of 1 Fe 55. 85 Fe(NO 3)2 contains. . . 2 N 14. 01 28. 02 1 iron atom 2 nitrogen atoms 6 O 16. 00 96. 00 6 oxygen atoms TOTAL MOLAR 179. 87 MASS

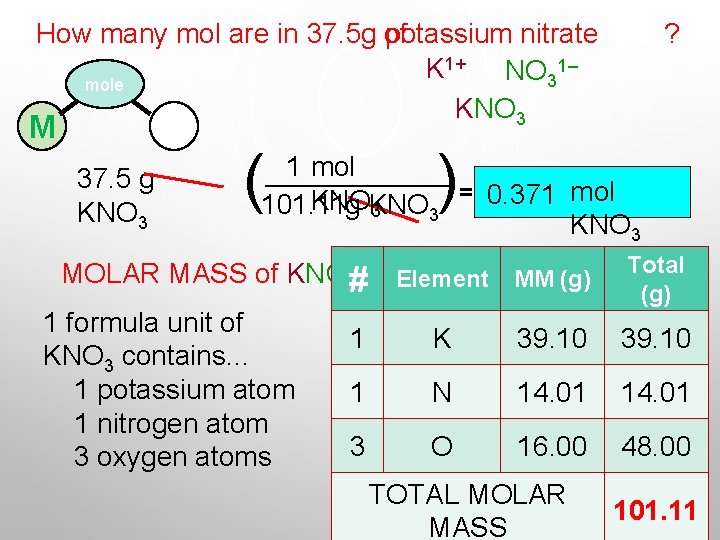
How many mol are in 37. 5 g of potassium nitrate 1+ 1– K NO 3 mole KNO 3 M P 37. 5 g KNO 3 ? ) ( 1 mol = 0. 371 mol KNO 101. 11 g KNO 3 3 KNO 3 MOLAR MASS of KNO 3# Element MM (g) 1 formula unit of KNO 3 contains. . . 1 potassium atom 1 nitrogen atom 3 oxygen atoms Total (g) 1 K 39. 10 1 N 14. 01 3 O 16. 00 48. 00 TOTAL MOLAR MASS 101. 11

PRACTICE WITH YOUR PARTNER 1. How many moles of magnesium are in 3. 01 x 1022 atoms? 2. How many molecules are there in 4. 00 moles of glucose? 3. How many moles in 28 grams of carbon dioxide? 4. What is the mass of 5 moles of Fe 2 O 3? 5. How many (and what type of) particles of N 2 O 5 are in 13. 4 grams? 6. What is the mass of 3. 29 x 1026 particles (what type? ) of argon gas?
 Testimone filologia
Testimone filologia Idiografo filologia
Idiografo filologia Meme neguito
Meme neguito The mole and avogadro's law worksheet answers
The mole and avogadro's law worksheet answers Periodic table avogadro
Periodic table avogadro What is a mole
What is a mole Stp temperature
Stp temperature Molar mass
Molar mass Stoichiometry mole-mole problems
Stoichiometry mole-mole problems Stoichiometry mole-mole
Stoichiometry mole-mole Mole mole factor
Mole mole factor How to convert mass to moles
How to convert mass to moles Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Mole-mass-volume relationships
Mole-mass-volume relationships Natalia galindo memes
Natalia galindo memes