HAHS Avogadro The Mole and Grams The Mole





- Slides: 14

HAHS Avogadro, The Mole, and Grams

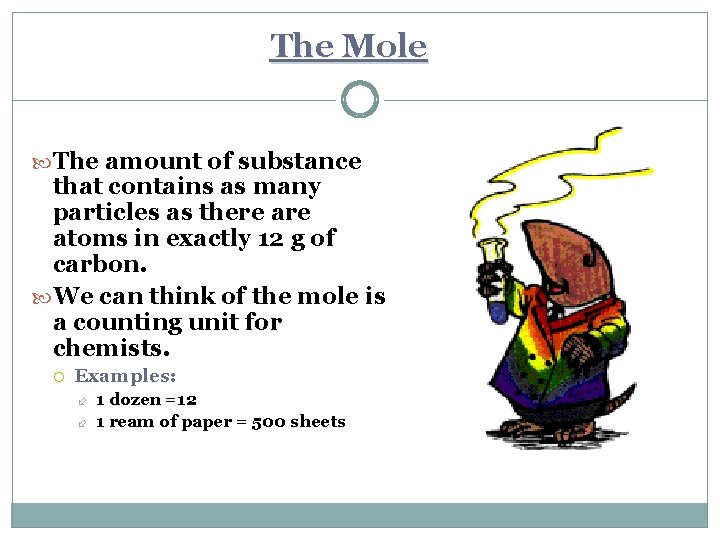
The Mole The amount of substance that contains as many particles as there atoms in exactly 12 g of carbon. We can think of the mole is a counting unit for chemists. Examples: 1 dozen =12 1 ream of paper = 500 sheets

Avogadro’s Number Is the number of particles in exactly one mole of a pure substance. 6. 02 x 1023 is called “Avogadro’s Number” in honor of the Italian chemist Amadeo Avogadro (1776 -1855). I didn’t discover it. Its just named after me!

Avogadro’s Number (con’t) There is 60200000000000 atoms in 1 mole of a substance THAT’S A GIGANTIC NUMBER!!!!!

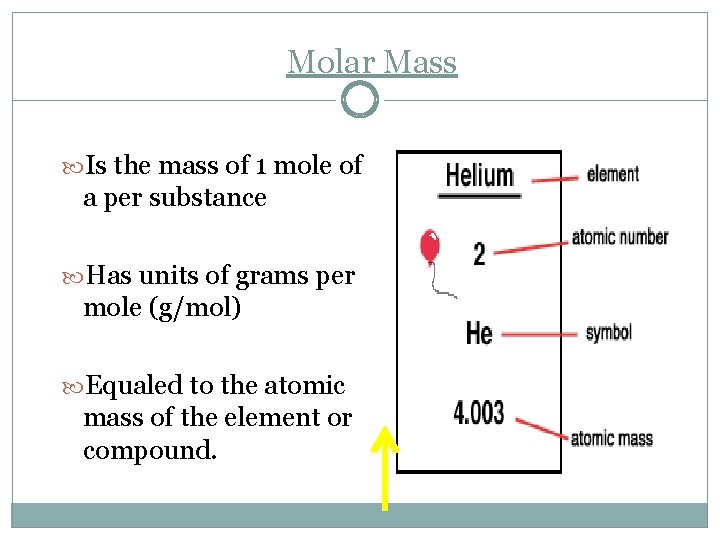
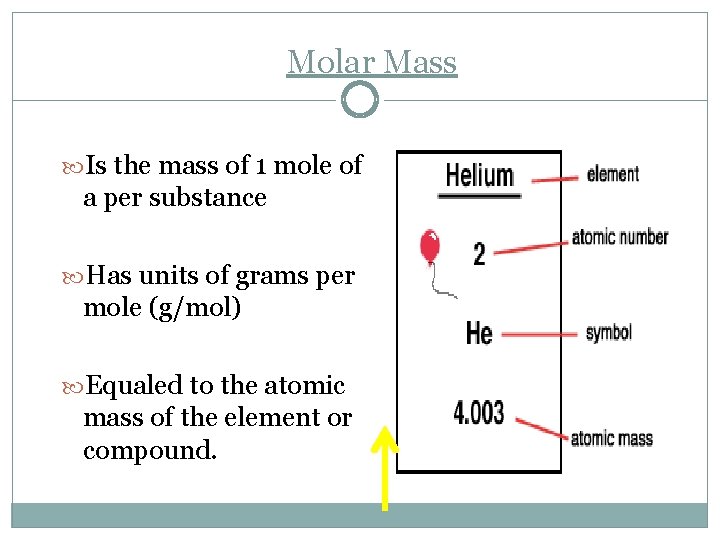
Molar Mass Is the mass of 1 mole of a per substance Has units of grams per mole (g/mol) Equaled to the atomic mass of the element or compound.

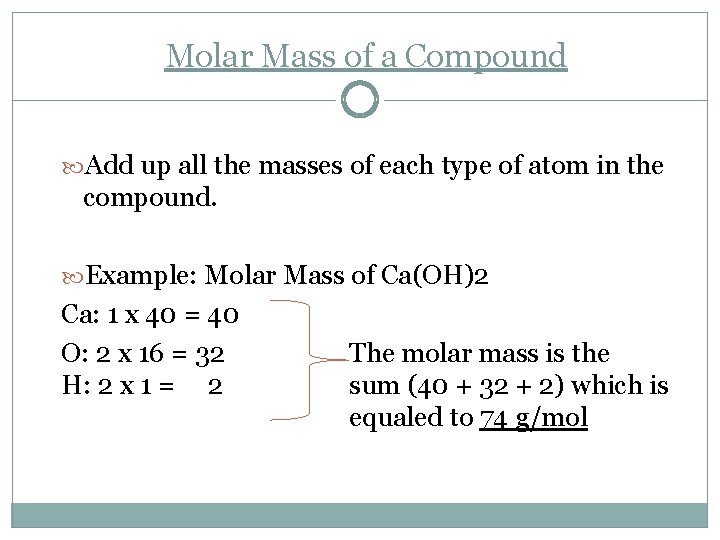
Molar Mass of a Compound Add up all the masses of each type of atom in the compound. Example: Molar Mass of Ca(OH)2 Ca: 1 x 40 = 40 O: 2 x 16 = 32 H: 2 x 1 = 2 The molar mass is the sum (40 + 32 + 2) which is equaled to 74 g/mol

Molar Mass Is the mass of 1 mole of a per substance Has units of grams per mole (g/mol) Equaled to the atomic mass of the element or compound.

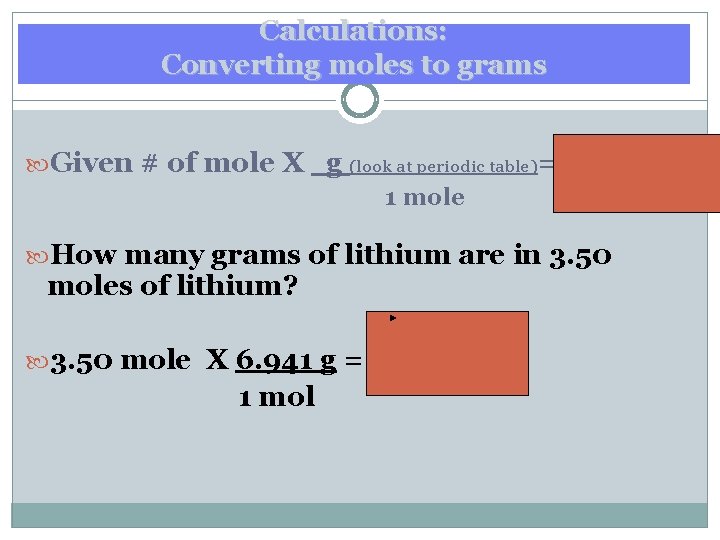
Calculations: Converting moles to grams Given # of mole X g (look at periodic table)= 1 mole g of How many grams of lithium are in 3. 50 moles of lithium? 3. 50 mole X 6. 941 g = 24. 29 g Li 1 mol

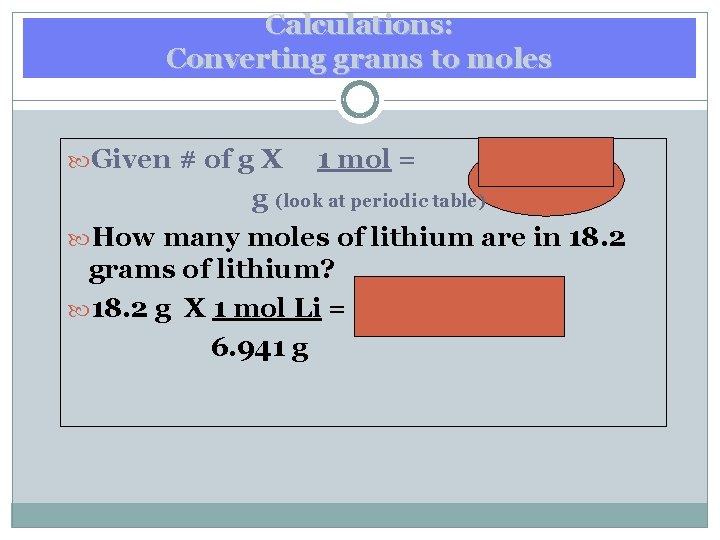
Calculations: Converting grams to moles Given # of g X 1 mol = mol of g (look at periodic table) How many moles of lithium are in 18. 2 grams of lithium? 18. 2 g X 1 mol Li = 2. 622 mol Li 6. 941 g

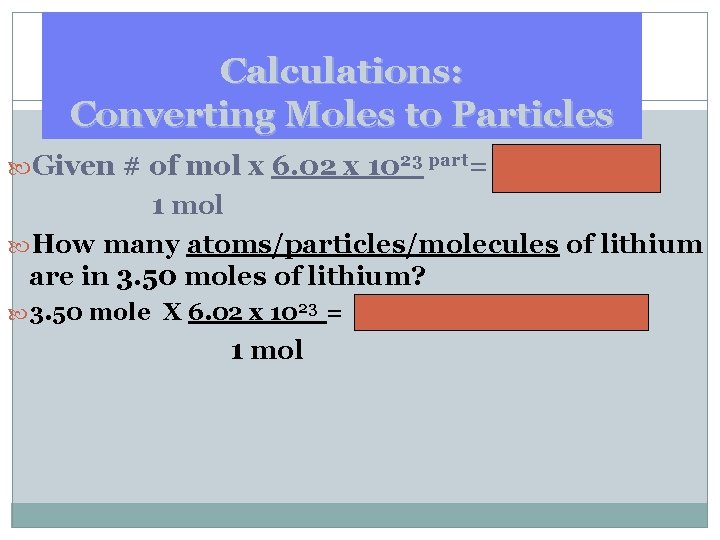
Calculations: Converting Moles to Particles Given # of mol x 6. 02 x 1023 part= atoms 1 mol How many atoms/particles/molecules of lithium are in 3. 50 moles of lithium? 3. 50 mole X 6. 02 x 1023 = 2. 11 x 1024 atoms of Li 1 mol

Calculations: Converting Particles to Moles Given # of particles x 1 mole = mol 6. 02 x 1023 How many moles of lithium are there in 1. 2044 x 1024 particles of Li? 1. 2044 x 1024 part x 1 mole = 2. 0 mol Li 6. 02 x 1023 part

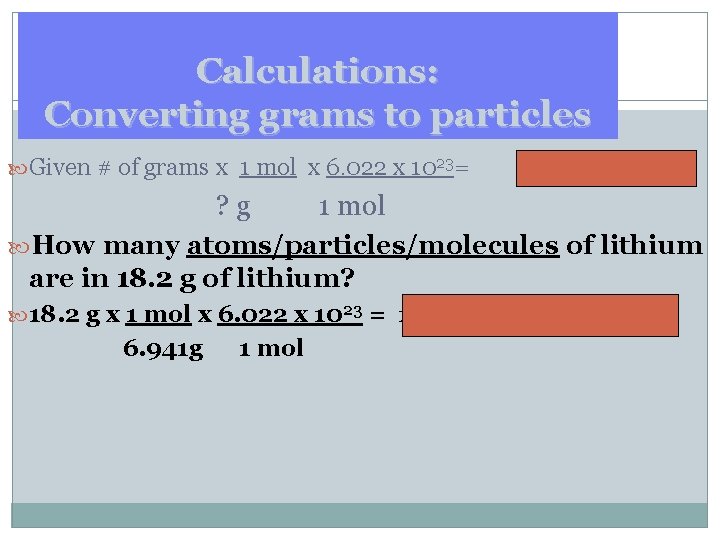
Calculations: Converting grams to particles Given # of grams x 1 mol x 6. 022 x 1023= particles ? g 1 mol How many atoms/particles/molecules of lithium are in 18. 2 g of lithium? 18. 2 g x 1 mol x 6. 022 x 1023 = 1. 58 x 1024 particle Li 6. 941 g 1 mol

Calculations: Converting particles to grams Given # of particles x 1 mol x ? g= g 6. 022 x 1023 1 mol 25 particles How many grams are there in 8. 02 x 10 How many grams of lithium are in 3. 50 moles of lithium?

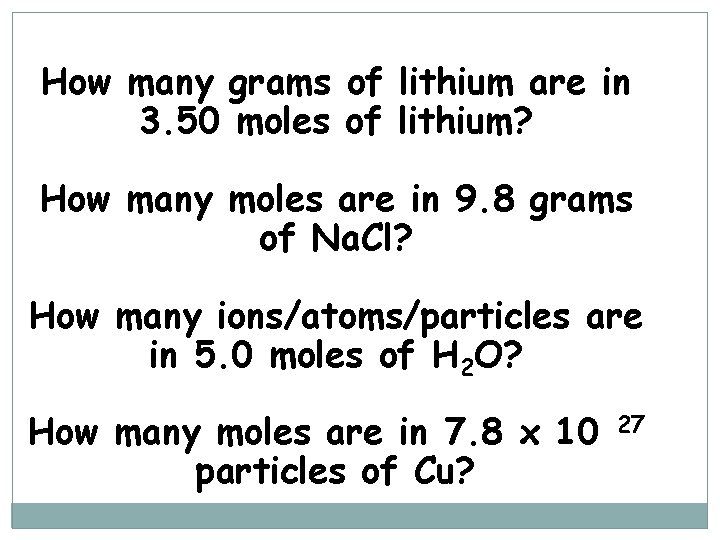
How many grams of lithium are in 3. 50 moles of lithium? How many moles are in 9. 8 grams of Na. Cl? How many ions/atoms/particles are in 5. 0 moles of H 2 O? How many moles are in 7. 8 x 10 particles of Cu? 27
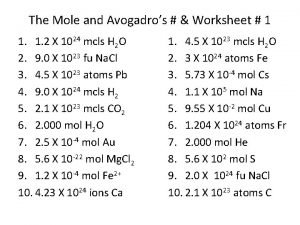
 The mole and avogadro's worksheet
The mole and avogadro's worksheet What is avogadro's number used for
What is avogadro's number used for One mole of pennies
One mole of pennies Stp temperature
Stp temperature How many grams in a mole
How many grams in a mole How to convert mass to moles
How to convert mass to moles Mol from mass
Mol from mass Mole road map chemistry
Mole road map chemistry Volume to mols
Volume to mols Concentration moles and volume
Concentration moles and volume Mole-mass-volume relationships
Mole-mass-volume relationships Gram to gram conversion
Gram to gram conversion Mole-mole factor
Mole-mole factor Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Molar mass of sucrose
Molar mass of sucrose