The Mole The Mole o Mole amount of




















- Slides: 41

The Mole

The Mole o Mole– amount of substance that contains the same # of atoms in exactly 12 grams of carbon-12 o Since we cannot count the number of atoms, mass is our link to find the number of atoms o The number of particles in a mole has been experimentally determined in a number of ways

The Mole There are three types of mole conversions: Moles Liters (volume) Moles Particles (atoms, molecules, formula units) Moles Grams (mass)

The Mole To perform mole conversions, you must use the 5 -step method: I. Given II. Looking For III. Conversion Factor IV. Dimensional Analysis Grid V. Answer (boxed with units and sig figs)

The Mole Once you have your answer, make sure to box it with the correct number of significant figures (the same as your given) EX: Your given is 0. 56 moles, which has two significant figures; therefore, your answer should have two significant figures Also make sure you include your unit, whether moles, grams, liters, or particles (atoms/ molecules/ formula units) NO WORK = NO CREDIT

The Mole Significant Figure Review: o All non-zero numbers are significant o Trapped zeroes are significant o Leading zeroes are NOT significant o Trailing zeroes are only significant if there is a decimal somewhere

Moles Liters

Moles Liters Each mole of a gas contains 22. 4 L at STP (standard temperature and pressure) standard temperature = 0°C / 273 Kelvins (K) standard pressure = 1 atmosphere (atm) Conversion Factor: 1 mole = 22. 4 liters (L)

Moles Liters How many liters are in 2. 38 moles of oxygen?

Moles Liters How many moles are in 16. 0 liters of NO 2 (g)?

Moles Particles

Moles Particles Particle – atoms, formula units, and molecules Atom if pure element EX: K Formula unit if ionic compound (+ -) EX: Na. Cl Molecule if molecular compound (- -) EX: P 7 O 3

Moles Particles Avagadro’s Number = 6. 022137 x 1023 o This is the # of particles in exactly one mole of a pure substance o For most purposes, Avagadro’s Number is usually rounded to 6. 02 x 1023 Conversion Factor: 1 mole = 6. 02 x 1023 particles

Moles Particles How many atoms are in 0. 61 moles of copper?

Moles Particles How many moles are in 2. 34 x 1013 formula units of potassium iodide?

Moles Particles How many molecules are in 4. 8 moles of carbon monoxide?

Moles Grams

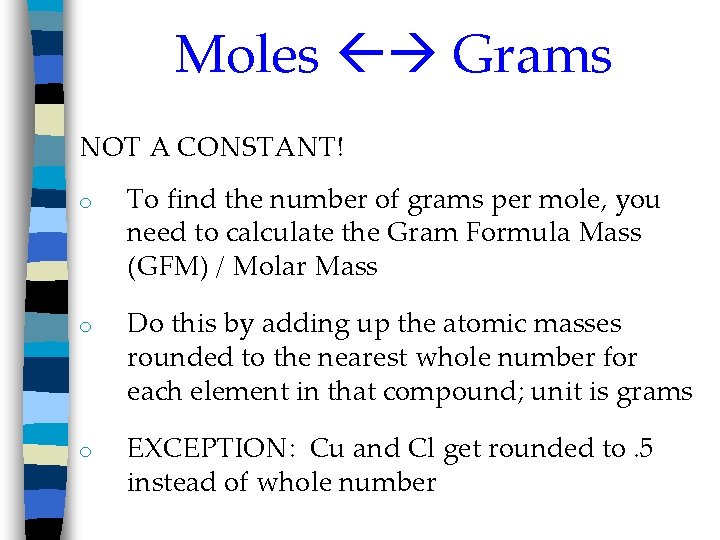
Moles Grams NOT A CONSTANT! o To find the number of grams per mole, you need to calculate the Gram Formula Mass (GFM) / Molar Mass o Do this by adding up the atomic masses rounded to the nearest whole number for each element in that compound; unit is grams o EXCEPTION: Cu and Cl get rounded to. 5 instead of whole number

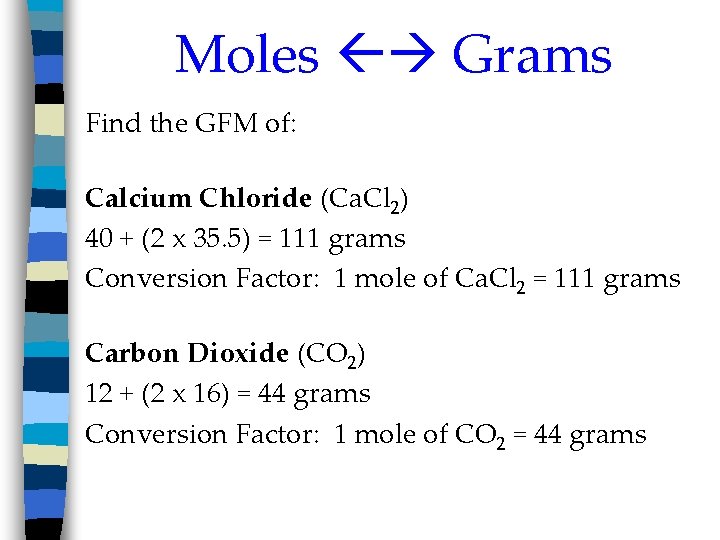
Moles Grams Find the GFM of: Calcium Chloride (Ca. Cl 2) 40 + (2 x 35. 5) = 111 grams Conversion Factor: 1 mole of Ca. Cl 2 = 111 grams Carbon Dioxide (CO 2) 12 + (2 x 16) = 44 grams Conversion Factor: 1 mole of CO 2 = 44 grams

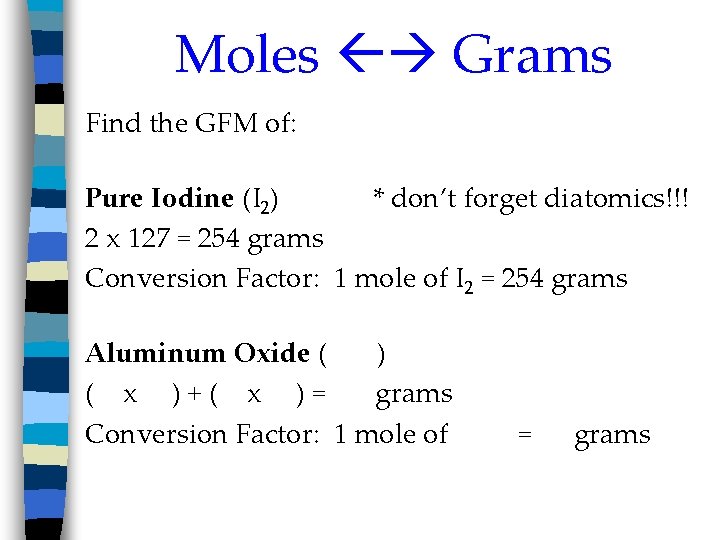
Moles Grams Find the GFM of: Pure Iodine (I 2) * don’t forget diatomics!!! 2 x 127 = 254 grams Conversion Factor: 1 mole of I 2 = 254 grams Aluminum Oxide ( ) ( x )+( x )= grams Conversion Factor: 1 mole of = grams

Moles Grams How many grams are in 0. 56 moles of potassium sulfate?

Moles Grams How many moles are in 127. 3 grams of difluorine pentanitride?

2 -Step Problems

2 -Step Problems How many liters are in 42 grams of oxygen gas?

2 -Step Problems How many grams are in 5. 20 x 108 molecules of carbon trifluoride?

Percent Composition

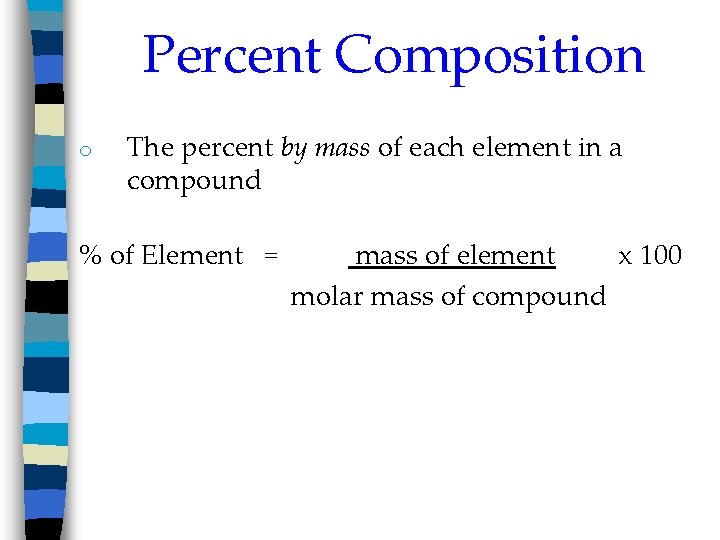
Percent Composition o The percent by mass of each element in a compound % of Element = mass of element x 100 molar mass of compound

Percent Composition Find the percent composition of sodium in sodium hydroxide (Na. OH).

Percent Composition Find the percent composition of each element in phosphoric acid.

Percent Composition Find the moles of H+ in 25. 0 m. L of 0. 200 M H 2 SO 4.

Percent Composition Find the number of moles of OH- in 0. 04 L of 0. 300 M calcium hydroxide.

Empirical & Molecular Formulas

Empirical Formulas n The empirical formula is simply the simplest whole number ratio of the elements in a compound. n The molecular formula is the actual number of atoms that makes up the molecule For example: n the empirical formula of ethene (C 2 H 4) is CH 2 n the empirical formula of butene (C 4 H 8) is CH 2

Empirical Formulas n Calculate empirical formula of a compound that is 85. 5% carbon and 14. 5% hydrogen. First, assume that you have 100 g of the substance, so you really have 85. 5 g of C and 14. 5 g of H. Next, convert it to moles using molar mass. Finally, find the lowest whole number ratio between the two elements.

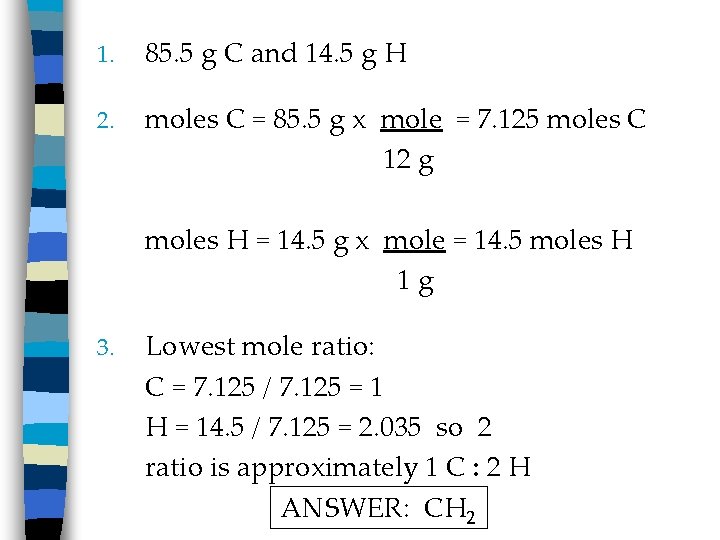
1. 85. 5 g C and 14. 5 g H 2. moles C = 85. 5 g x mole = 7. 125 moles C 12 g moles H = 14. 5 g x mole = 14. 5 moles H 1 g 3. Lowest mole ratio: C = 7. 125 / 7. 125 = 1 H = 14. 5 / 7. 125 = 2. 035 so 2 ratio is approximately 1 C : 2 H ANSWER: CH 2

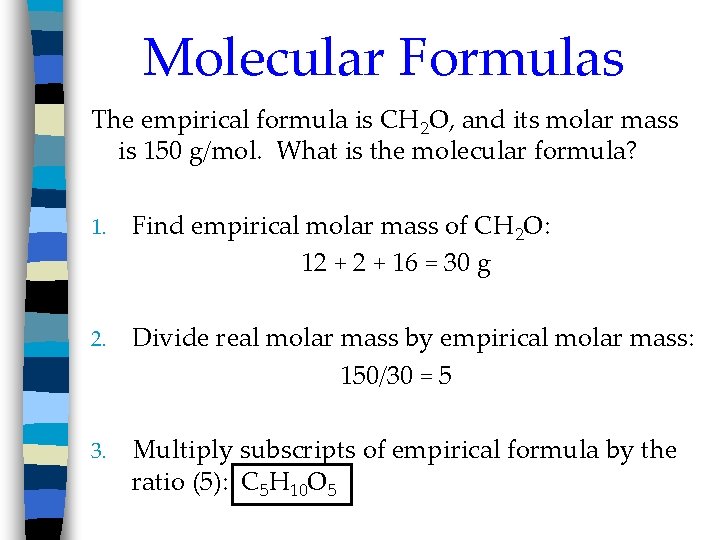
Molecular Formulas The empirical formula is CH 2 O, and its molar mass is 150 g/mol. What is the molecular formula? 1. Find empirical molar mass of CH 2 O: 12 + 16 = 30 g 2. Divide real molar mass by empirical molar mass: 150/30 = 5 3. Multiply subscripts of empirical formula by the ratio (5): C 5 H 10 O 5

Molarity

Molarity o Method of determining the concentration of solute in a solution Solute: what is being dissolved Solvent: substance into which the solute is dissolved Solution: homogenous mixture of 2+ substances Molarity = moles of solute liters of solution moles = M (unit) L * Reminder: 1 Liter = 1, 000 milliliters (m. L)

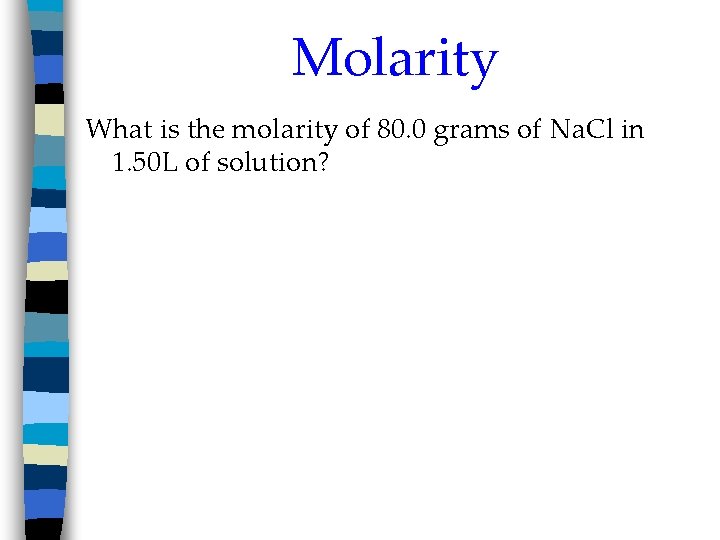
Molarity What is the molarity of 80. 0 grams of Na. Cl in 1. 50 L of solution?

Molarity How many moles are in 45. 0 m. L of a 0. 200 M solution of Ba. SO 4?

Molarity How many grams are contained in 4. 00 L of 2. 50 M Na. OH?