Mole Recap What is a mole A mole
















- Slides: 21

Mole Recap

What is a mole? • A mole is a very large number of atoms or molecules • 6. 02 x 10 23

What we know. . • ma= relative atomic mass - sometimes also written as Ar • n = number of moles (mol) • M = molar mass (g/mol) • NA = Avogadros constant • N = number of molecules or atoms

What was Avogadro thinking. . • Given the relative atomic mass, ma, a carbon atom must be 12 times heavier than a hydrogen atom etc. • From the periodic table • Therefore, if he took 12 times more mass of carbon than hygrogen (12 g vs. 1 g ) he should have the same amount of atoms. • 16 g of oxygen, 12 g of carbon, 1 g of hydrogen should all have the same amount of atoms. • Then he counted this amount of atoms. . . 6. 02 x 10 23 and named it the Avogadro constant NA or the mole.

Formula – to convert number of atoms to moles n (mol) = N NA n = moles N = number of aoms or molecules present NA = Avogadros constant = 6. 02 x 10 23

Worked example • Calculate the amount in moles of carbn dioxide in a sample of 1. 50 x 1023 molecules. n = N /NA n (CO 2) = 1. 5 x 1023 / 6. 02 x 1023 N (CO 2) = 0. 249 mol

Try these now Calculate the amount of molecules of H 2 O in 0. 236 moles. • Calculate the number of carbon atoms contained in 1. 50 mol of glucose C 6 H 12 O 6

Answer • N = n x NA =. 0. 236 x NA = 1. 421 x 1023 • 1 mole of glucose conatines 6 atoms of carbon, 12 atoms of H and 6 atoms of O • 1 mole of glucose contains 6 moles of C • 1. 5 mole of glucose contains 9 moles of C • N = 9 x NA • N = 5. 42 x 1024 C atoms

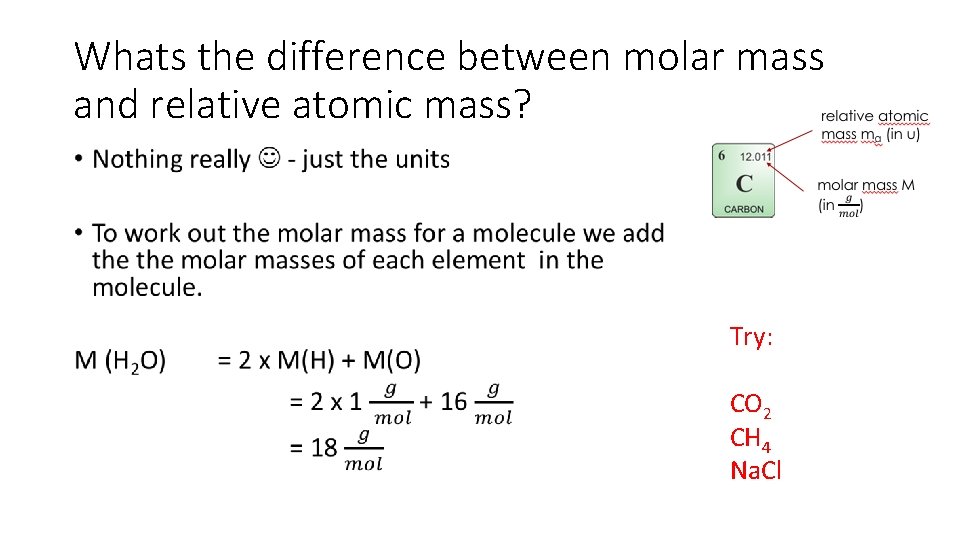
Whats the difference between molar mass and relative atomic mass? • Try: CO 2 CH 4 Na. Cl

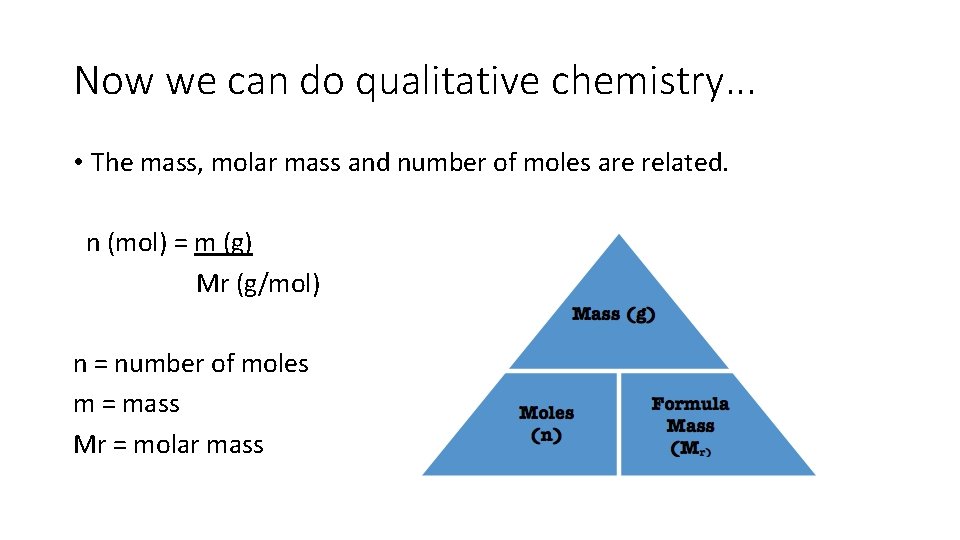
Now we can do qualitative chemistry. . . • The mass, molar mass and number of moles are related. n (mol) = m (g) Mr (g/mol) n = number of moles m = mass Mr = molar mass

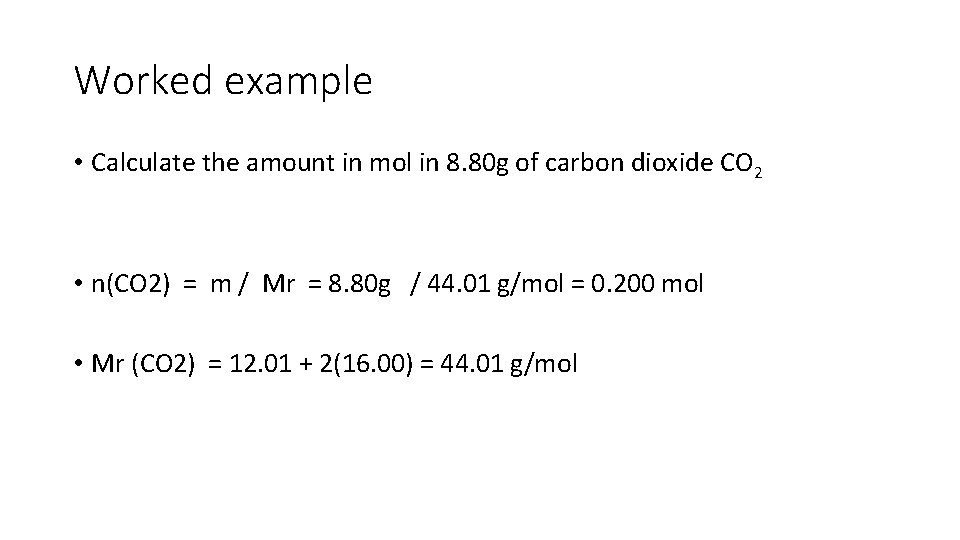
Worked example • Calculate the amount in mol in 8. 80 g of carbon dioxide CO 2 • n(CO 2) = m / Mr = 8. 80 g / 44. 01 g/mol = 0. 200 mol • Mr (CO 2) = 12. 01 + 2(16. 00) = 44. 01 g/mol

Try these now • Calculate the amount (in mol) of the following masses. • 8. 09 g of Aluminium, Al • 9. 8 g of sulfuric acid H 2 SO 4

Answers • n (Al)= m/Mr • n (Al) = 8. 09 g / 26. 98 g/mol = 0. 30 mol • n(H 2 SO 4) =. m/Mr. = 9. 8 / 98. 03. = 0. 10 mol • Mr (H 2 SO 4) = 2 x 1. 01. +. 32. 01. +. 4 x 16. 00 = 98. 03

Molar concentrations •

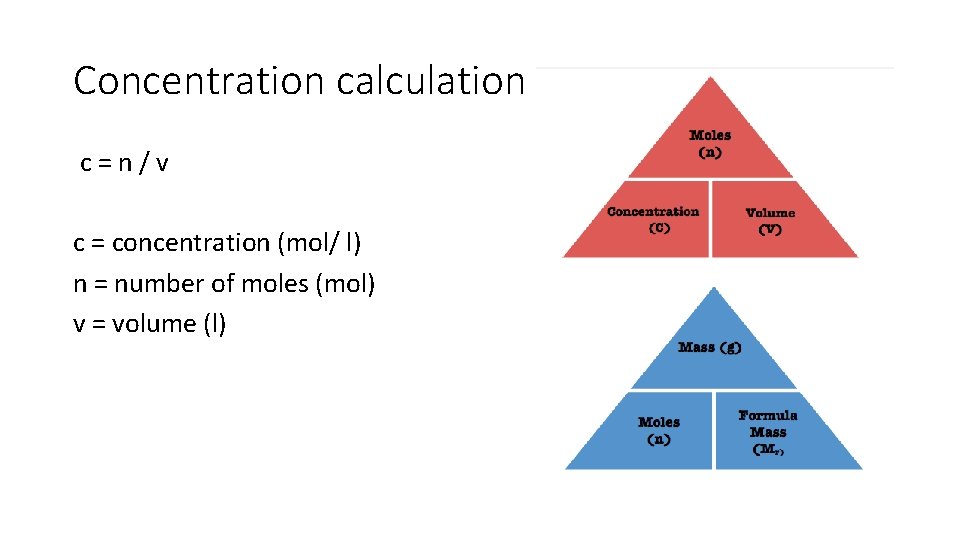
Concentration calculation c=n/v c = concentration (mol/ l) n = number of moles (mol) v = volume (l)

Molar concentration •

Try these •

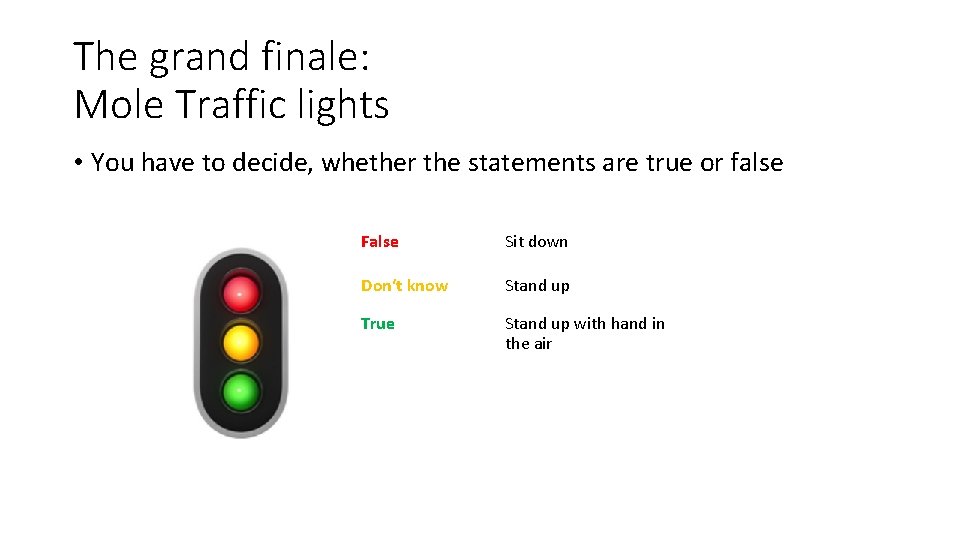
The grand finale: Mole Traffic lights • You have to decide, whether the statements are true or false False Sit down Don‘t know Stand up True Stand up with hand in the air

The grand finale: Mole Traffic lights • The mole is a unit • The mole is a mass • One mole of water (H 2 O) and one mole of glucose (C 6 H 12 O 6) have the same mass • One mole of H 2 contains the same number of molecules as one mole of CO 2 • One mole of H 2 contains the same number of atoms as one mole of CO 2 • There are more atoms in 12 g of carbon than in 12 g of copper

The grand finale: Mole Traffic lights • A mole is a molecule • The mole is a number • One mole of a substance contains Avogadro‘s number of atoms or molecules • One mole of a substance contains 6, 02 x 1023 atoms or molecules • The amount of a substance is measured in kilograms • The amount of substance is a volume • I understand what a mole is
