The Mole Not this kind Or this kind










- Slides: 13

The Mole Not this kind Or this kind

But First, An Analogy If I ask you to go get 3 dozen eggs, that’s 36 eggs. 1 dozen = 12 This is not exactly a unit; it’s a grouping that modifies a unit. I still need to tell you 3 dozen eggs, or 2 dozen bagels; eggs and bagels are the actual unit. All I’ve done is put them into groups of 12 to make counting easier.

Back to the Mole A mole is a grouping just like pair (2), or dozen (12) 1 mole = 6. 022*1023 (this is called Avogadro’s number) Ok, so that’s a lot of stuff. We can have a mole of anything: bagels, eggs, walruses. (that’s a lot of walruses) We can have two moles of bagels, in which case we have 120440000000000 bagels (12. 044*1023) Probably, this is not a useful grouping for bagels, eggs, or really much of anything else you can see.

Definitely not Walruses Physics on a mole of moles: http: //what-if. xkcd. com/4/ Walruses would presumably be about the same.

But What About Atoms? This is what one mole of carbon atoms (graphite allotrope) looks like. Counting out atoms or molecules in groups of moles is a lot more reasonable. (In dozens, this is still 50183000000000 dozen atoms. Dozens will not be a very useful grouping here)

Doing Math With Moles Just do it as unit conversion, where 1 mole = 6. 022*1023 is your conversion factor. Example: If I have 4. 35 moles of sodium, how many atoms is that? 4. 35 moles Na * 6. 022*1023 atoms Na 1 mole Na = 2. 62 * 1024 atoms Na Note: see how I was careful to include moles of Na, and atoms of Na? That is a good habit—it will be relevant later. Example 2: If I have 8. 52*1027 atoms of silicon, how many moles of silicon do I have? 8. 52*1027 atoms Si * 1 mole Si 6. 022*1023 atoms Si = 14100 moles Si

Yes, you can have decimals There’s no reason you can’t have a half dozen bagels, right? Same thing with moles: 1. 81*1023 atoms Si * 1 mole Si 6. 022*1023 atoms Si = 0. 301 moles Si

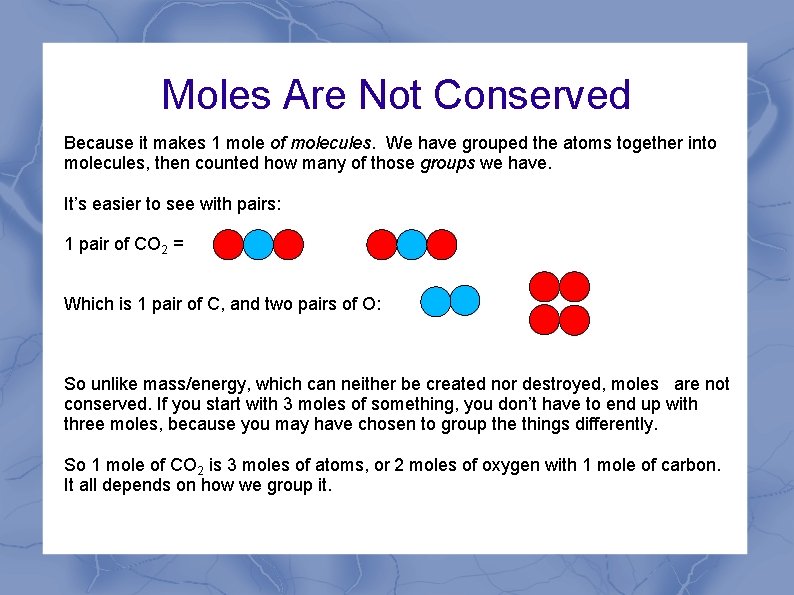
Moles Are Not Conserved Let’s say that I have 1 mole of CO 2 molecules. Therefore, I have 6. 022*1023 CO 2 molecules. If each of those is made of one carbon and two oxygens, then I have: Which is 6. 022*1023 carbon atoms and 12. 044*1023 oxygen atoms 1 mole carbon atoms 2 moles of oxygen atoms and So how can 1 mole of atoms with 2 moles of atoms make 1 mole?

Moles Are Not Conserved Because it makes 1 mole of molecules. We have grouped the atoms together into molecules, then counted how many of those groups we have. It’s easier to see with pairs: 1 pair of CO 2 = Which is 1 pair of C, and two pairs of O: So unlike mass/energy, which can neither be created nor destroyed, moles are not conserved. If you start with 3 moles of something, you don’t have to end up with three moles, because you may have chosen to group the things differently. So 1 mole of CO 2 is 3 moles of atoms, or 2 moles of oxygen with 1 mole of carbon. It all depends on how we group it.

So Why This Awful Number? Why 6. 022*1023? Wouldn’t 1*1023 or 1*1024 make more sense, and have the advantage of being metric? YES! But…

So Why This Awful Number? Mass of a proton/neutron = 1. 6726*10 -24 g Mass of 1 mole of protons/neutrons: 6. 022*1023 protons * 1. 6726*10 -24 g/proton = 1 g !!! I bet this will turn out to be relevant….

Why Moles At All? 1. Numbers of atoms are so huge that even scientific notation gets annoying. Writing “ 2 moles Na” is much simpler than “ 1. 2044*1024 atoms Na” 2. That thing about 1 mole of protons weighing 1 gram This is a mole cricket. Real thing. I promise. Totally not made up.

Summary • Moles are a grouping, not a unit themselves • 1 mole = 6. 022*1023 things (Avogadro’s number) • Moles are not conserved, because you can group things in different ways, which changes how many of them there are. • 1 mole of protons/neutrons weighs 1 gram. • Nature is full of terrifying animals.