Quantitative Chemistry Activity Print out the keywords worksheet










































- Slides: 95

Quantitative Chemistry

Activity: Print out the keywords worksheet and add the following words, once you know the meanings fill them in. Relative atomic mass Molecule Relative molecular mass Mole Relative formula mass Isotope Avogadro’s number Diatomic Element Compound

Chemical Formulas and the Mole concept Elements Compounds Mole concept and Avogadro's constant Isotopes Formulas of compounds Empirical formula Molecular formula Structural formula

Elements All substances are made up of one or more _____. An element ______be broken down by any chemical process into ______substances. There are just over _____ known elements. The smallest part of an element is called an _____. cannot 100 elements atom simpler

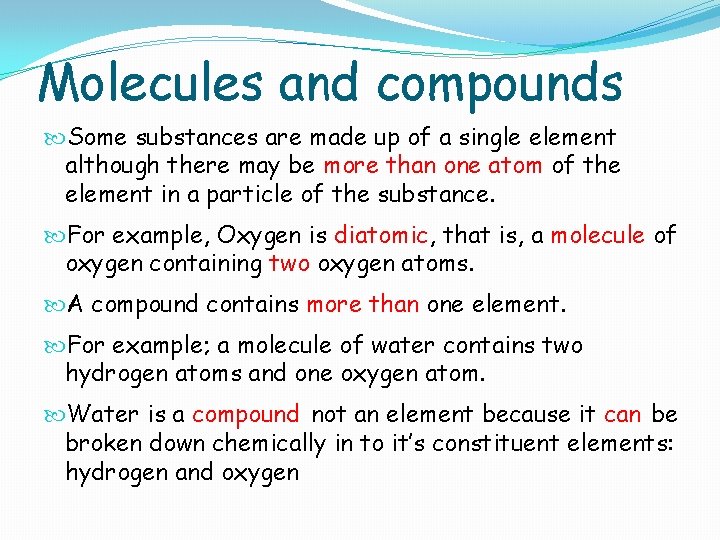
Molecules and compounds Some substances are made up of a single element although there may be more than one atom of the element in a particle of the substance. For example, Oxygen is diatomic, that is, a molecule of oxygen containing two oxygen atoms. A compound contains more than one element. For example; a molecule of water contains two hydrogen atoms and one oxygen atom. Water is a compound not an element because it can be broken down chemically in to it’s constituent elements: hydrogen and oxygen

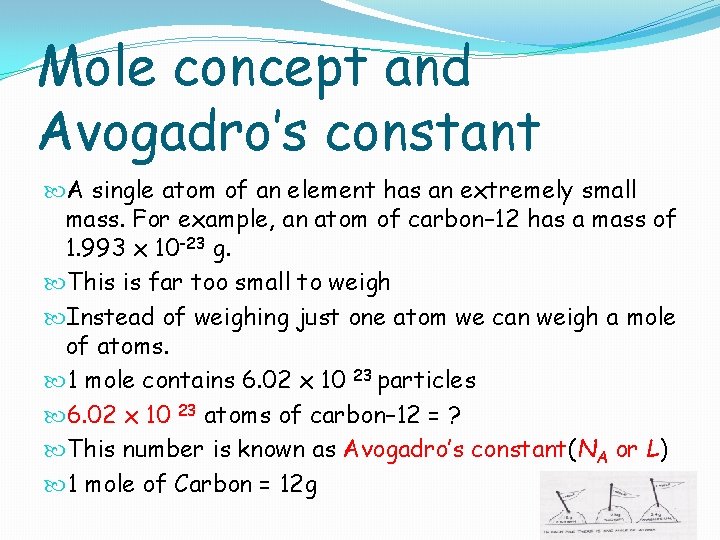
Mole concept and Avogadro’s constant A single atom of an element has an extremely small mass. For example, an atom of carbon– 12 has a mass of 1. 993 x 10 -23 g. This is far too small to weigh Instead of weighing just one atom we can weigh a mole of atoms. 1 mole contains 6. 02 x 10 23 particles 6. 02 x 10 23 atoms of carbon– 12 = ? This number is known as Avogadro’s constant(NA or L) 1 mole of Carbon = 12 g

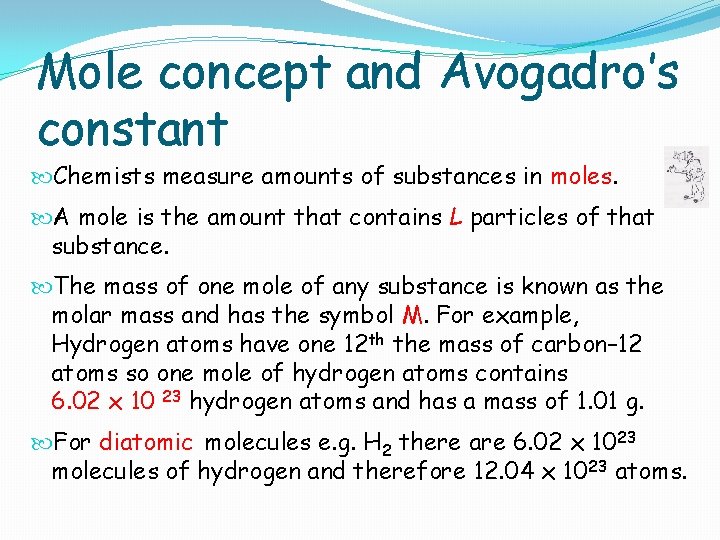
Mole concept and Avogadro’s constant Chemists measure amounts of substances in moles. A mole is the amount that contains L particles of that substance. The mass of one mole of any substance is known as the molar mass and has the symbol M. For example, Hydrogen atoms have one 12 th the mass of carbon– 12 atoms so one mole of hydrogen atoms contains 6. 02 x 10 23 hydrogen atoms and has a mass of 1. 01 g. For diatomic molecules e. g. H 2 there are 6. 02 x 1023 molecules of hydrogen and therefore 12. 04 x 1023 atoms.

Relative Atomic mass The actual atomic mass of an individual atom of an element is so small that we use a relative atomic mass as seen on the periodic table. Since hydrogen is the lightest atom (it has an actual mass of 8. 275 x 10 -25) we say that hydrogen has a relative atomic mass of 1. Experiments have shown that an atom of carbon weighs 12 times as much as an atom of hydrogen. So the relative atomic mass of carbon is 12. Relative atomic mass can be abbreviated to Ar or RAM and is found on the periodic table. It has no units.

Activity Complete the worksheet IB 1. 1 – Using Avogadro’s Number There are 20 questions to complete Show all working

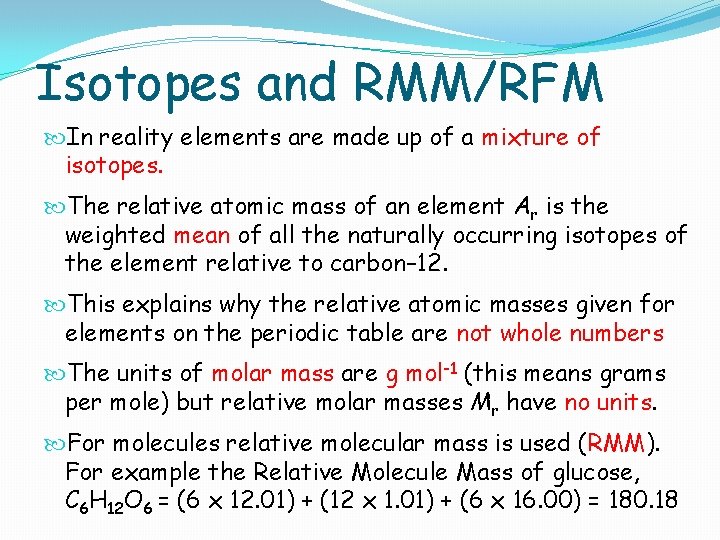
Isotopes and RMM/RFM In reality elements are made up of a mixture of isotopes. The relative atomic mass of an element Ar is the weighted mean of all the naturally occurring isotopes of the element relative to carbon– 12. This explains why the relative atomic masses given for elements on the periodic table are not whole numbers The units of molar mass are g mol-1 (this means grams per mole) but relative molar masses Mr have no units. For molecules relative molecular mass is used (RMM). For example the Relative Molecule Mass of glucose, C 6 H 12 O 6 = (6 x 12. 01) + (12 x 1. 01) + (6 x 16. 00) = 180. 18

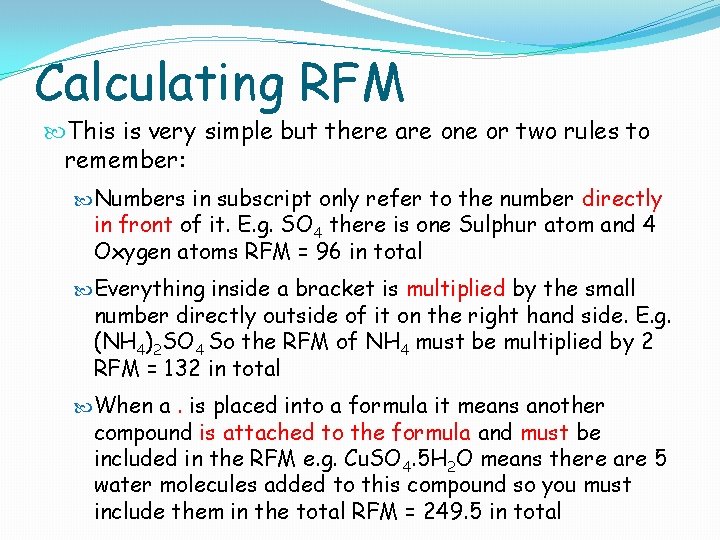
Calculating RFM This is very simple but there are one or two rules to remember: Numbers in subscript only refer to the number directly in front of it. E. g. SO 4 there is one Sulphur atom and 4 Oxygen atoms RFM = 96 in total Everything inside a bracket is multiplied by the small number directly outside of it on the right hand side. E. g. (NH 4)2 SO 4 So the RFM of NH 4 must be multiplied by 2 RFM = 132 in total When a. is placed into a formula it means another compound is attached to the formula and must be included in the RFM e. g. Cu. SO 4. 5 H 2 O means there are 5 water molecules added to this compound so you must include them in the total RFM = 249. 5 in total

Activity Complete the worksheet IB 1. 2 - Calculation of the Molar Mass of Compounds There are 60 questions to complete Don’t forget to refer to the previously stated rules Show all working

Using Molar masses As we have seen 1 g of Hydrogen contains many more atoms than 1 g of carbon. Calculate the number of atoms in 1 g of hydrogen and 1 g of carbon. Suggest why we cannot use mass as a means of keeping a fair test during an experiment i. e. The reaction of substances with oxygen which would give out more energy 1 g of hydrogen or 1 g of carbon? To keep a test fair we use molar quantities.

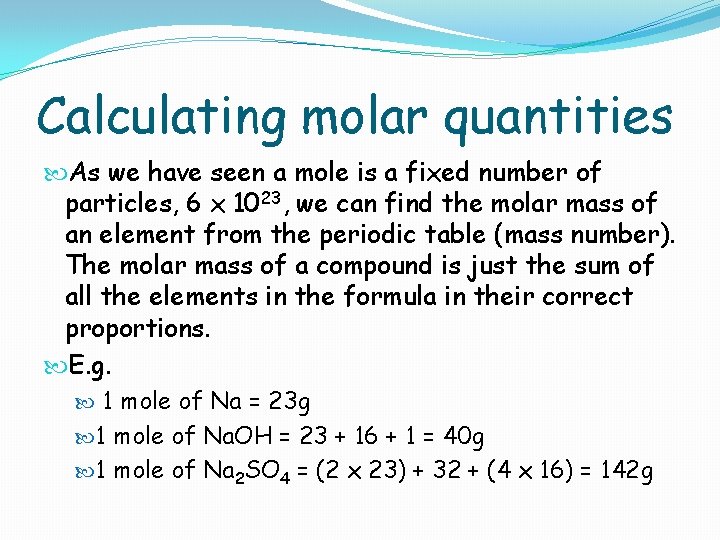
Calculating molar quantities As we have seen a mole is a fixed number of particles, 6 x 1023, we can find the molar mass of an element from the periodic table (mass number). The molar mass of a compound is just the sum of all the elements in the formula in their correct proportions. E. g. 1 mole of Na = 23 g 1 mole of Na. OH = 23 + 16 + 1 = 40 g 1 mole of Na 2 SO 4 = (2 x 23) + 32 + (4 x 16) = 142 g

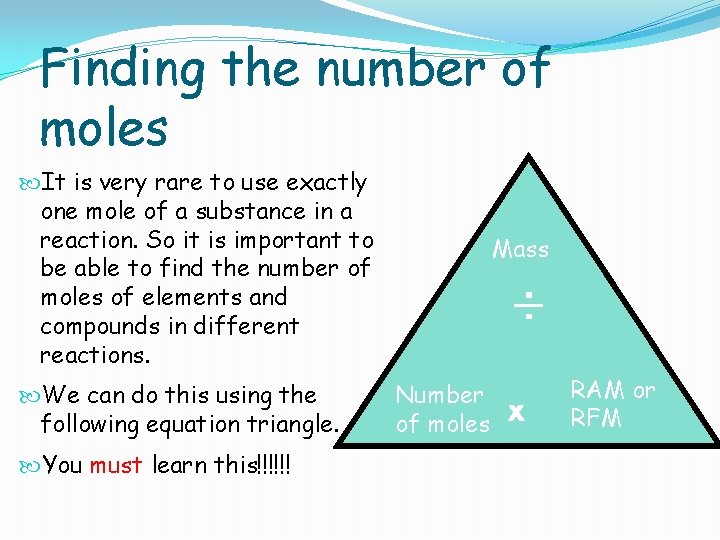
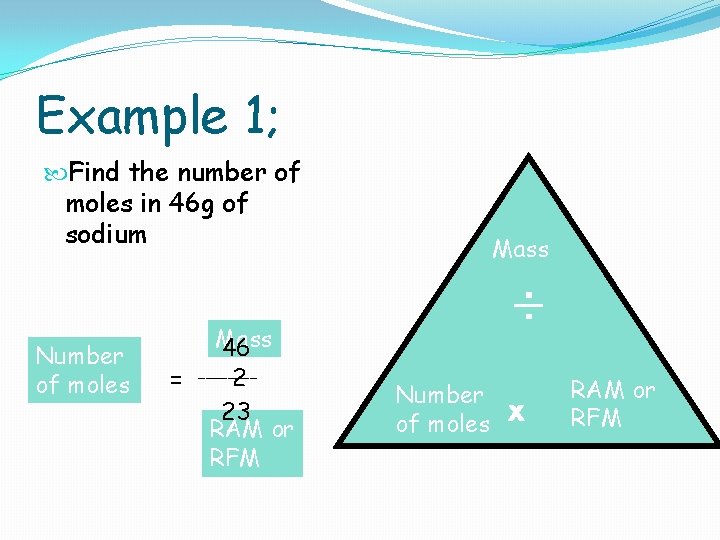
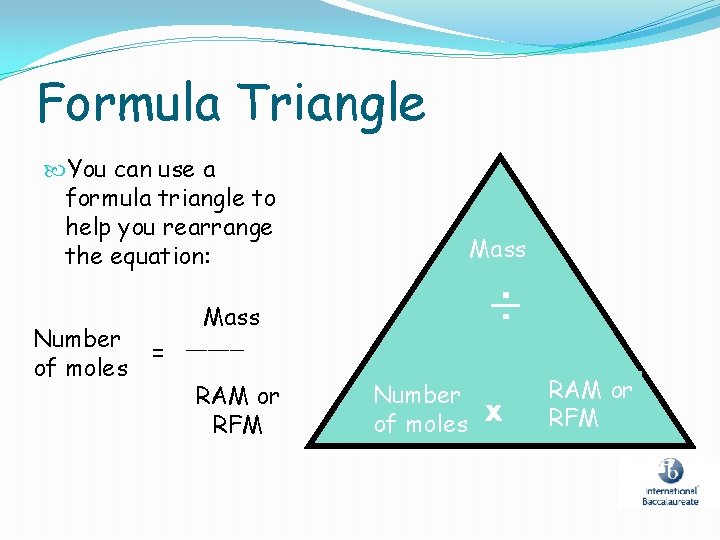
Finding the number of moles It is very rare to use exactly one mole of a substance in a reaction. So it is important to be able to find the number of moles of elements and compounds in different reactions. We can do this using the following equation triangle. You must learn this!!!!!! Mass ÷ Number of moles x RAM or RFM

Example 1; Find the number of moles in 46 g of sodium Number of moles Mass 46 2 = ____ 23 RAM or RFM Mass ÷ Number of moles x RAM or RFM

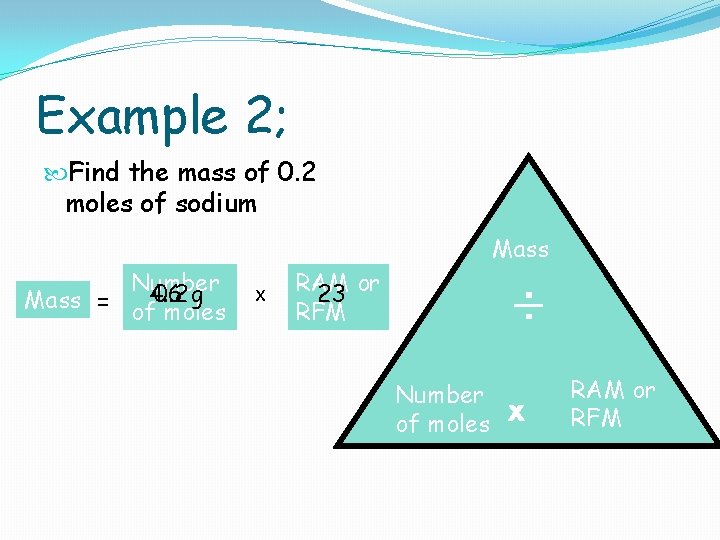
Example 2; Find the mass of 0. 2 moles of sodium Mass Number 0. 2 g Mass = of 4. 6 moles x RAM 23 or RFM ÷ Number of moles x RAM or RFM

Activity Complete the worksheet IB 1. 3– Molar calculations and IB 1. 4 Molar calculations (II) There are 60 questions to complete Don’t forget to refer to the previously stated rules Show all working

Formula’s of compounds Compounds can be described by different chemical formulas: Empirical Molecular Structural

Empirical Formula Literally this is the formula obtained by experiment. This shows the simplest whole number ratio of atoms of each element in a particle of the substance It can be obtained by either knowing the mass of each element in the compound or from the percentage composition by mass of the compound. The percentage composition can be converted directly into mass by assuming 100 g of the compound are taken.

Formula Triangle You can use a formula triangle to help you rearrange the equation: Number of moles Mass ÷ Mass = ____ RAM or RFM Number of moles x RAM or RFM

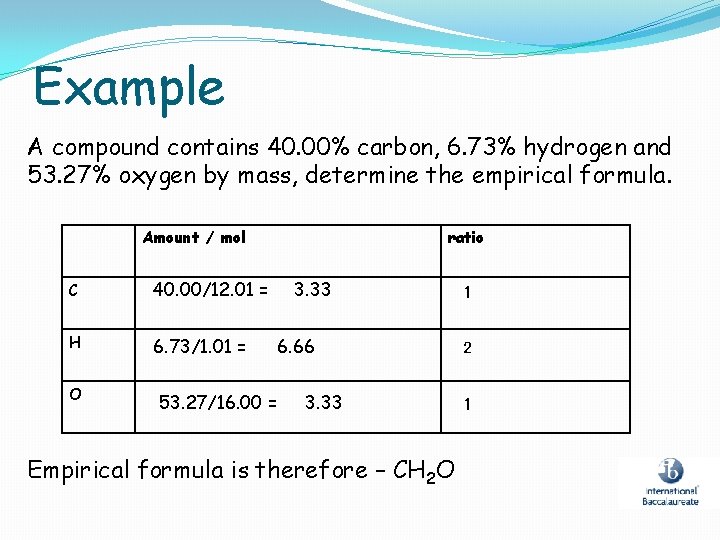
Example A compound contains 40. 00% carbon, 6. 73% hydrogen and 53. 27% oxygen by mass, determine the empirical formula. Amount / mol C 40. 00/12. 01 = H 6. 73/1. 01 = O 53. 27/16. 00 = ratio 3. 33 6. 66 3. 33 Empirical formula is therefore – CH 2 O 1 2 1

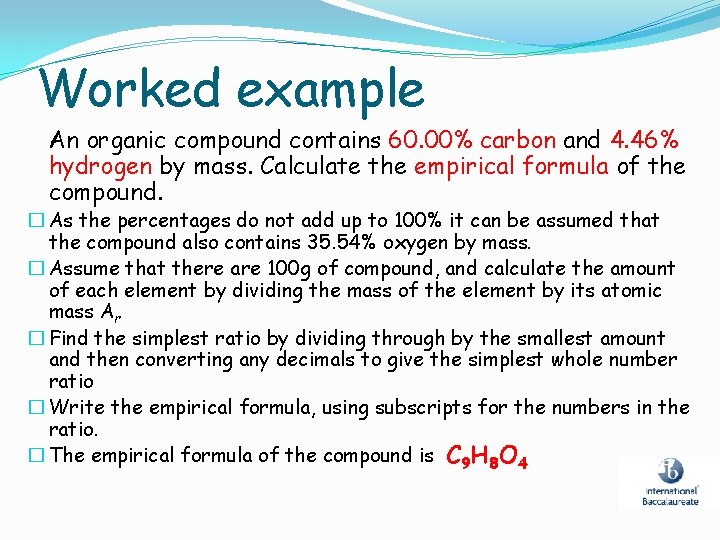
Worked example An organic compound contains 60. 00% carbon and 4. 46% hydrogen by mass. Calculate the empirical formula of the compound. � As the percentages do not add up to 100% it can be assumed that the compound also contains 35. 54% oxygen by mass. � Assume that there are 100 g of compound, and calculate the amount of each element by dividing the mass of the element by its atomic mass Ar � Find the simplest ratio by dividing through by the smallest amount and then converting any decimals to give the simplest whole number ratio � Write the empirical formula, using subscripts for the numbers in the ratio. � The empirical formula of the compound is C 9 H 8 O 4

Activity; Read through the examples 16, 17 and 18 on pages 31 and 32 in ‘Calculations in AS/A Level Chemistry’ – Jim Clark Complete problems 15 and 16 part a only. Answers are at the back of the book page 279 Complete worksheet 1. 5 Formulae and Percentage composition

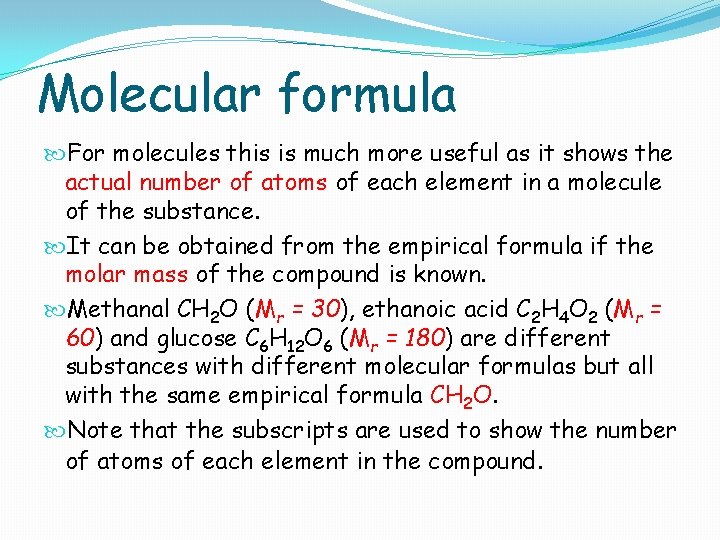
Molecular formula For molecules this is much more useful as it shows the actual number of atoms of each element in a molecule of the substance. It can be obtained from the empirical formula if the molar mass of the compound is known. Methanal CH 2 O (Mr = 30), ethanoic acid C 2 H 4 O 2 (Mr = 60) and glucose C 6 H 12 O 6 (Mr = 180) are different substances with different molecular formulas but all with the same empirical formula CH 2 O. Note that the subscripts are used to show the number of atoms of each element in the compound.

Activity; Using your answers from part a (from last lesson)complete problems 15 and 16 part b only. Answers are at the back of the book page 279 Complete worksheet; Worksheet IB 1. 6 Section BMolecular Formulae

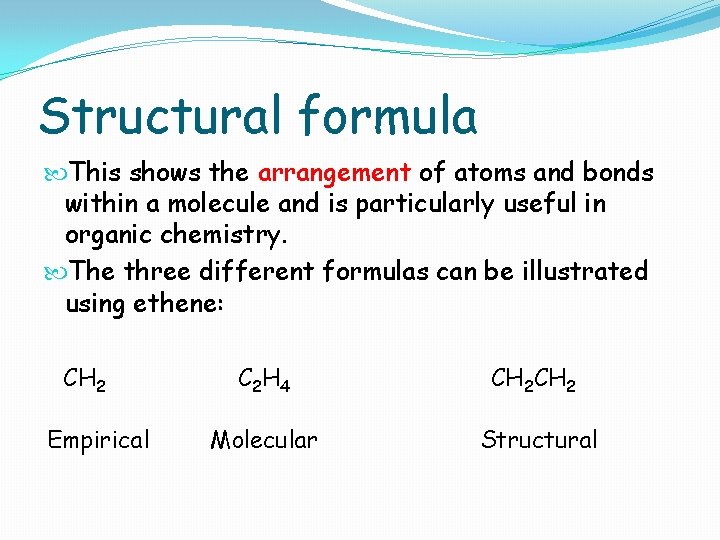
Structural formula This shows the arrangement of atoms and bonds within a molecule and is particularly useful in organic chemistry. The three different formulas can be illustrated using ethene: CH 2 Empirical C 2 H 4 CH 2 Molecular Structural

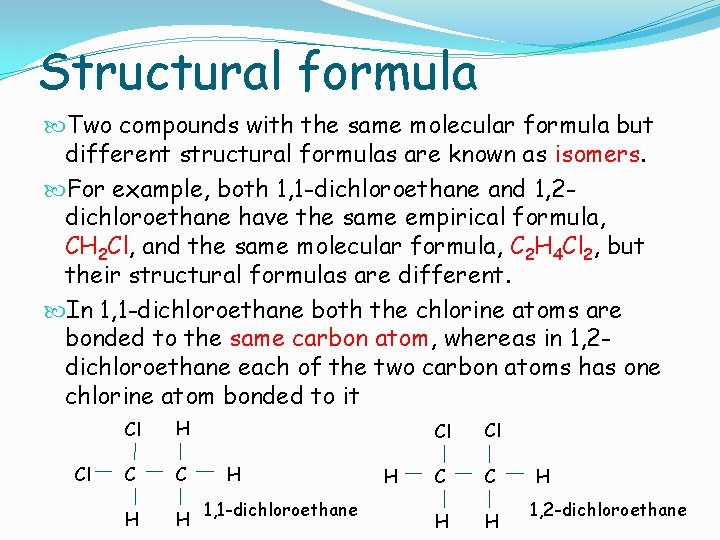
Structural formula Two compounds with the same molecular formula but different structural formulas are known as isomers. For example, both 1, 1 -dichloroethane and 1, 2 dichloroethane have the same empirical formula, CH 2 Cl, and the same molecular formula, C 2 H 4 Cl 2, but their structural formulas are different. In 1, 1 -dichloroethane both the chlorine atoms are bonded to the same carbon atom, whereas in 1, 2 dichloroethane each of the two carbon atoms has one chlorine atom bonded to it Cl Cl H C C H H H 1, 1 -dichloroethane H Cl Cl C C H H H 1, 2 -dichloroethane

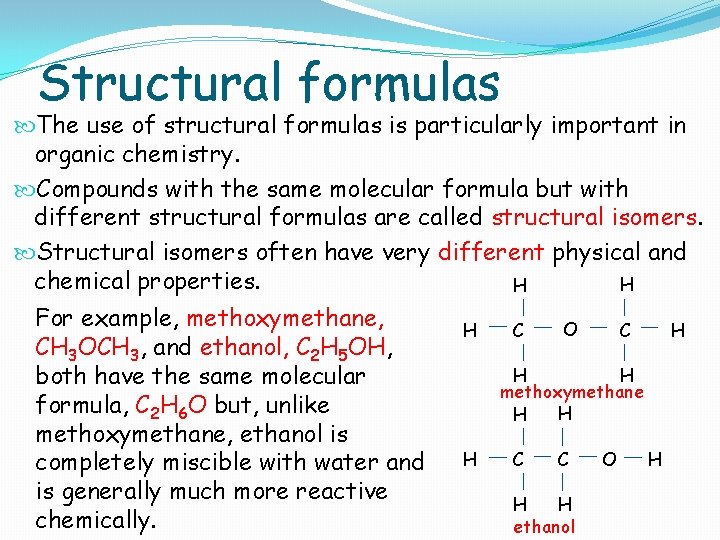
Structural formulas The use of structural formulas is particularly important in organic chemistry. Compounds with the same molecular formula but with different structural formulas are called structural isomers. Structural isomers often have very different physical and chemical properties. H H For example, methoxymethane, CH 3 OCH 3, and ethanol, C 2 H 5 OH, both have the same molecular formula, C 2 H 6 O but, unlike methoxymethane, ethanol is completely miscible with water and is generally much more reactive chemically. H C O C H H H methoxymethane H H H C C H H ethanol O H

Quick Questions 1. What is the empirical formula of the following compounds? You could try to name them too. A liquid containing 2. 0 g of hydrogen, 32. 1 g sulphur, and 64 g oxygen. b) A white solid containing 0. 9 g beryllium, 3. 2 g oxygen, and 0. 2 g hydrogen. c) A white solid containing 0. 234 g magnesium and 0. 710 g chlorine. a) 2. 3. 888 g magnesium ribbon was burnt completely in air and 6. 488 g of magnesium oxide were produced. How many moles of magnesium and of oxygen are present in 6. 488 g of magnesium oxide? b) What is the empirical formula of magnesium oxide? More to follow… a)

Just a few more! 3) What are the empirical formulae of the following molecules? a) Cyclohexane C 6 H 12 b) Dichloroethene C 2 H 2 Cl 2 c) Benzene C 6 H 6 4) Mr for ethane-1, 2 -diol is 62. 0. It is composed of carbon, hydrogen and oxygen in the ratio by moles of 1: 3: 1. What is its molecular formula?

Answers 1) 1 H 2 SO 4 sulphuric acid b) Be. O 2 H 2 which is Be(OH)2, beryllium hydroxide c) Mg. Cl 2 magnesium chloride a) 2) 1 0. 16 moles of each b) Mg. O a) 3) 1 CH 2 b) CHCl c) CH a) 4) C 2 H 6 O 2

Working out formulae for ionic compounds You can’t write equations until you can write formulae. People tend to remember the formulae for common covalent substances like water or carbon dioxide or methane, and will rarely need to work them out. That is not true of ionic compounds. You need to know the symbols and charges of the common ions and how to combine them into a formula.

The need for equal numbers of “pluses” and “minuses” Ions are atoms or groups of atoms which carry electrical charges, either positive or negative. Compounds are electrically neutral. In an ionic compound there must therefore be the right number of each sort of ion so that the total positive charge is exactly the same as the total negative charge. Obviously, then, if you are going to work out the formula, you need to know the charges on the ions.

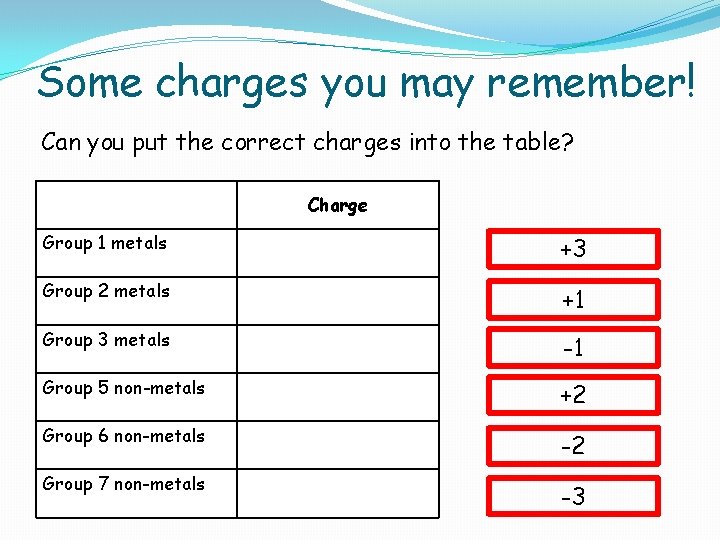
Some charges you may remember! Can you put the correct charges into the table? Charge Group 1 metals +3 Group 2 metals +1 Group 3 metals -1 Group 5 non-metals +2 Group 6 non-metals -2 Group 7 non-metals -3

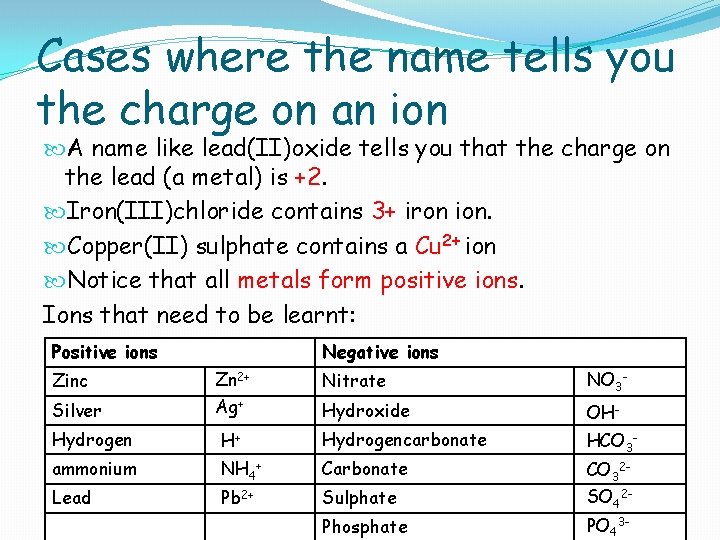
Cases where the name tells you the charge on an ion A name like lead(II)oxide tells you that the charge on the lead (a metal) is +2. Iron(III)chloride contains 3+ iron ion. Copper(II) sulphate contains a Cu 2+ ion Notice that all metals form positive ions. Ions that need to be learnt: Positive ions Negative ions Zinc Zn 2+ Nitrate Silver Ag+ NO 3 - Hydroxide OHHCO 3 - Hydrogen H+ Hydrogencarbonate ammonium NH 4+ Carbonate Lead Pb 2+ Sulphate SO 42 - Phosphate PO 43 - CO 32 -

Confusing endings Do not confuse ions like Try naming the sulphate with sulphide. following compounds: Mg 3 N 2 A name like sodium sulphide means that it Mg(NO 3)2 contains sodium and Ca. C 2 sulphur only. Ca. CO 3 Once you have an “ate” ending it means that there is something else there as well – often, but not always, oxygen

Activity Fill in the worksheet; Worksheet IB 1. 6 Important ions for IB calculations how many can you complete without looking them up? Click here for answers.

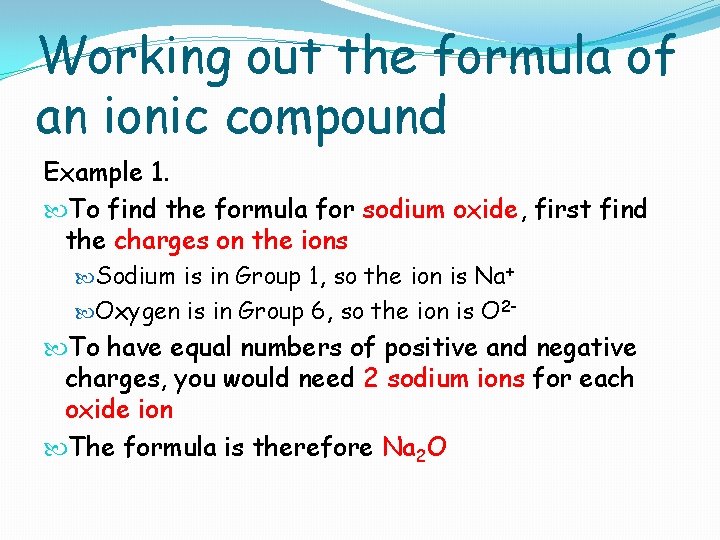
Working out the formula of an ionic compound Example 1. To find the formula for sodium oxide, first find the charges on the ions Sodium is in Group 1, so the ion is Na+ Oxygen is in Group 6, so the ion is O 2 - To have equal numbers of positive and negative charges, you would need 2 sodium ions for each oxide ion The formula is therefore Na 2 O

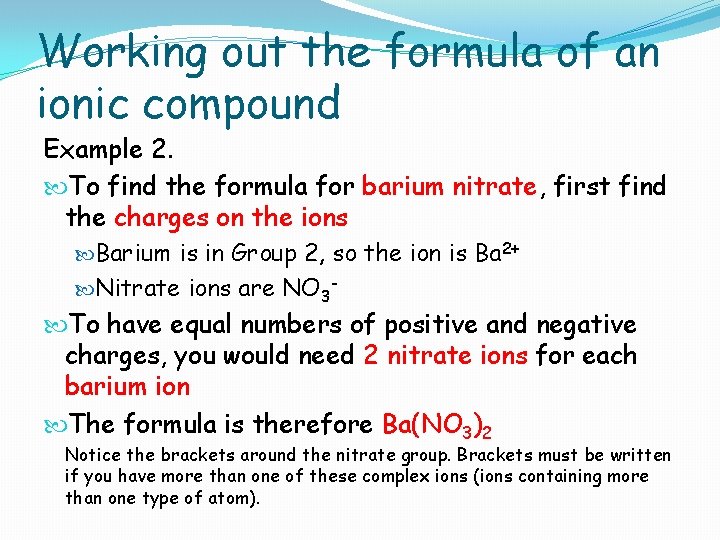
Working out the formula of an ionic compound Example 2. To find the formula for barium nitrate, first find the charges on the ions Barium is in Group 2, so the ion is Ba 2+ Nitrate ions are NO 3 - To have equal numbers of positive and negative charges, you would need 2 nitrate ions for each barium ion The formula is therefore Ba(NO 3)2 Notice the brackets around the nitrate group. Brackets must be written if you have more than one of these complex ions (ions containing more than one type of atom).

Working out the formula of an ionic compound Example 3. To find the formula for iron(III)sulphate, first find the charges on the ions Iron(III) tells us that the ion is Fe 3+ Sulphate ions are SO 42 - To have equal numbers of positive and negative charges, you would need 2 iron (III) ions for every 3 sulphate ions The formula is therefore Fe 2(SO 4)3

Activity Complete the worksheet; Worksheet IB 1. 7 Writing formulae from names For answers click here

Chemical Reactions and equations Properties of chemical reactions Chemical equations State symbols One way or reversible reactions Ionic equations

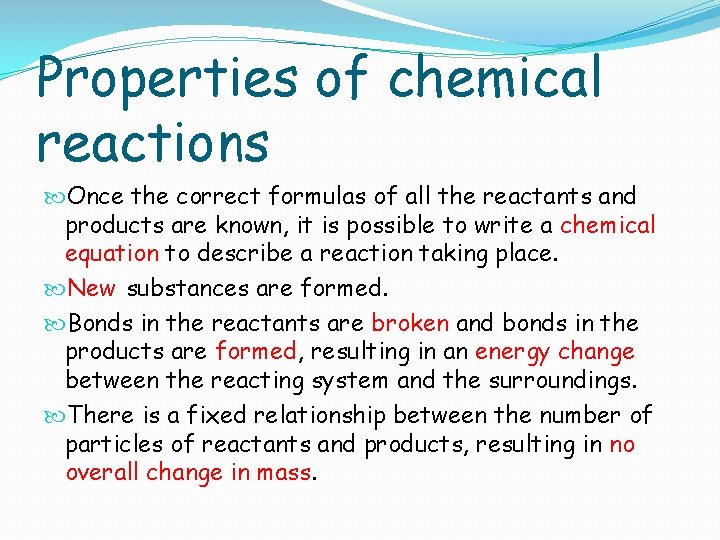
Properties of chemical reactions Once the correct formulas of all the reactants and products are known, it is possible to write a chemical equation to describe a reaction taking place. New substances are formed. Bonds in the reactants are broken and bonds in the products are formed, resulting in an energy change between the reacting system and the surroundings. There is a fixed relationship between the number of particles of reactants and products, resulting in no overall change in mass.

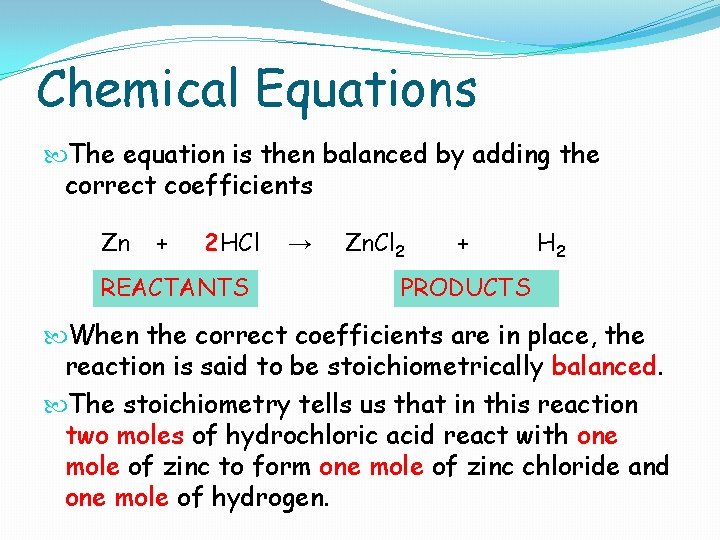
Chemical Equations In a chemical equation the reactants are written on the left hand side, and the products are written on the right hand side. As there is no overall change in mass, the total amount of each element must be the same on the two side of the equation. For example, consider the reaction between zinc metal and hydrochloric acid to produce zinc chloride and hydrogen gas. The correct formulas for all the reacting species and products are first written down. Zn + HCl REACTANTS → Zn. Cl 2 + PRODUCTS H 2

Chemical Equations The equation is then balanced by adding the correct coefficients Zn + 2 HCl REACTANTS → Zn. Cl 2 + H 2 PRODUCTS When the correct coefficients are in place, the reaction is said to be stoichiometrically balanced. The stoichiometry tells us that in this reaction two moles of hydrochloric acid react with one mole of zinc to form one mole of zinc chloride and one mole of hydrogen.

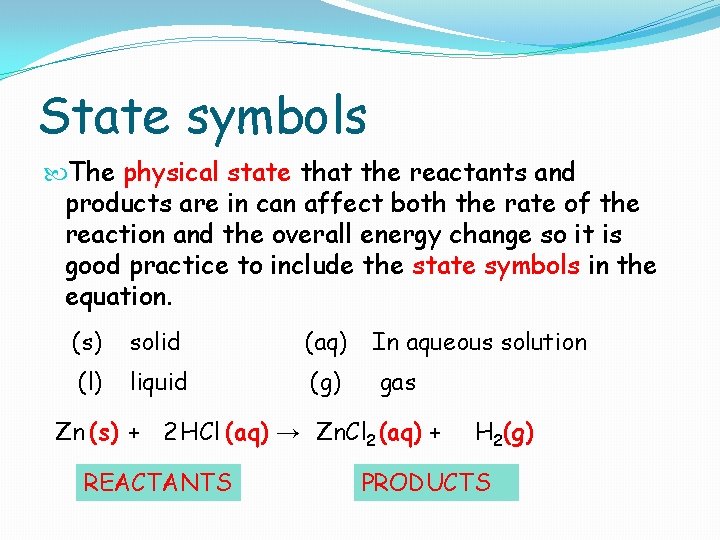
State symbols The physical state that the reactants and products are in can affect both the rate of the reaction and the overall energy change so it is good practice to include the state symbols in the equation. (s) solid (aq) In aqueous solution (l) liquid (g) gas Zn (s) + 2 HCl (aq) → Zn. Cl 2 (aq) + REACTANTS H 2(g) PRODUCTS

Activity Complete the worksheet; Worksheet IB 1. 8 Balancing equations For answers click here

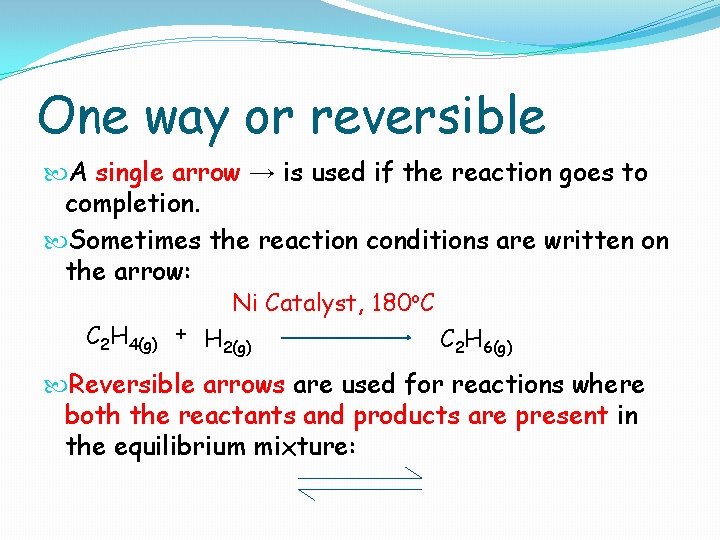
One way or reversible A single arrow → is used if the reaction goes to completion. Sometimes the reaction conditions are written on the arrow: Ni Catalyst, 180 o. C C 2 H 4(g) + H 2(g) C 2 H 6(g) Reversible arrows are used for reactions where both the reactants and products are present in the equilibrium mixture:

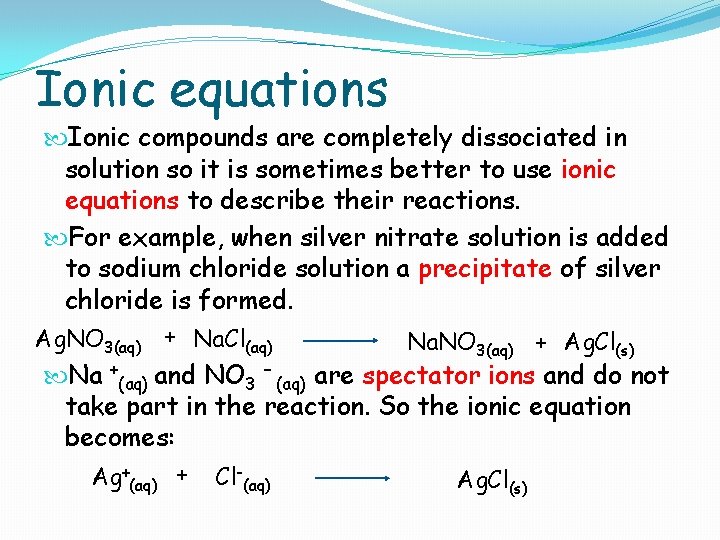
Ionic equations Ionic compounds are completely dissociated in solution so it is sometimes better to use ionic equations to describe their reactions. For example, when silver nitrate solution is added to sodium chloride solution a precipitate of silver chloride is formed. Ag. NO 3(aq) + Na. Cl(aq) Na. NO 3(aq) + Ag. Cl(s) Na +(aq) and NO 3 – (aq) are spectator ions and do not take part in the reaction. So the ionic equation becomes: Ag+(aq) + Cl-(aq) Ag. Cl(s)

Activity Carry out Experiment 1. 1 Precipitation reactions

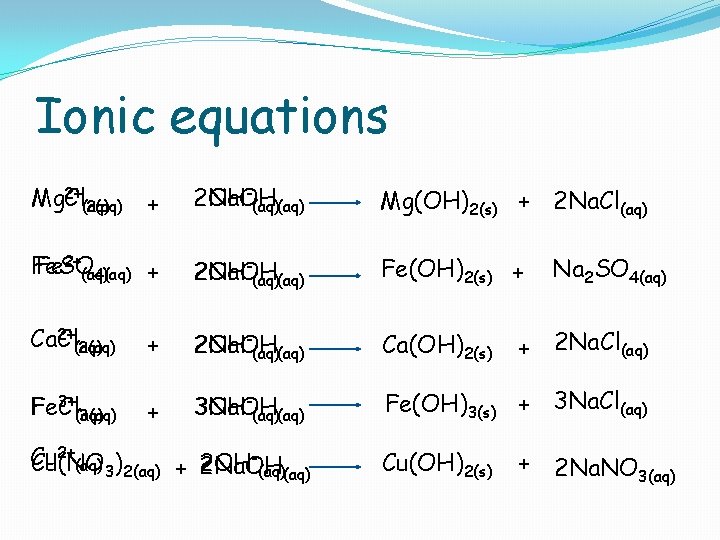
Precipitation Reactions Metal compound Metal ion Product ( Observation Mg. Cl 2(aq) Mg 2+ Mg(OH)2(s) White solid Fe. SO 4(aq) Fe 2+ Fe(OH)2(s) Dirty green solid Ca. Cl 2(aq) Ca 2+ Ca(OH)2(s) White solid Fe. Cl 3(aq) Fe 3+ Fe(OH)3(s) Rusty brown solid Cu(NO 3)2(aq) Cu 2+ Cu(OH)2(s) Blue solid To each of the following solutions add Na. OH solution drop-wise to see a precipitate form

Ionic equations 2+ Mg Mg. Cl (aq) 2(aq) + 2 Na. OH 2 OH-(aq) Mg(OH)2(s) + 2 Na. Cl(aq) Fe. SO Fe 2+(aq) 4(aq) + 2 OH 2 Na. OH (aq) Fe(OH)2(s) + 2+ Ca Ca. Cl (aq) 2(aq) + 2 OH 2 Na. OH (aq) Ca(OH)2(s) + 3 OH 3 Na. OH (aq) Fe(OH)3(s) + 3 Na. Cl(aq) 3+ Fe Fe. Cl (aq) 3(aq) 2+ Cu Cu(NO (aq) 3)2(aq) + 2 OH 2 Na. OH (aq) Cu(OH)2(s) Na 2 SO 4(aq) + 2 Na. Cl(aq) + 2 Na. NO 3(aq)

Measurements and calculations Error and uncertainty Measurements of molar quantities Solids Solutions Liquids Gases Worked examples

Error and uncertainty In the laboratory moles can conveniently be measured using either mass or volume depending on the substances involved. But with any measurement there is always uncertainty and error.

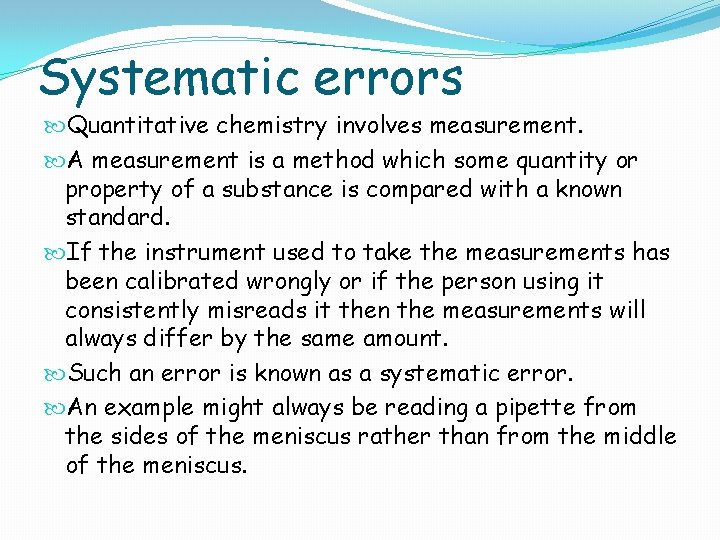
Systematic errors Quantitative chemistry involves measurement. A measurement is a method which some quantity or property of a substance is compared with a known standard. If the instrument used to take the measurements has been calibrated wrongly or if the person using it consistently misreads it then the measurements will always differ by the same amount. Such an error is known as a systematic error. An example might always be reading a pipette from the sides of the meniscus rather than from the middle of the meniscus.

Random uncertainties occur if there is an equal probability of the reading being too high or too low from one measurement to the next. These might include variations in the volume of glassware due to temperature fluctuations or the decision on exactly when an indicator changes colour during an acid base titration.

Precision and accuracy. Precision refers to how close several experimental measurements of the same quantity are to eachother. Accuracy refers to how close the readings are to the true value. This may the standard value,

Solids are normally measured by weighing to obtain the mass Masses are measured in grams and kilograms remember 1. 000 kg = 1000 g When weighing a substance the mass should be recorded to show the accuracy of the balance. For example, exactly 16 g of a substance would be recorded as 16. 00 g on a balance weighing to + or – 0. 01 g but as 16. 000 g on a balance weighing to + or – 0. 001 g.

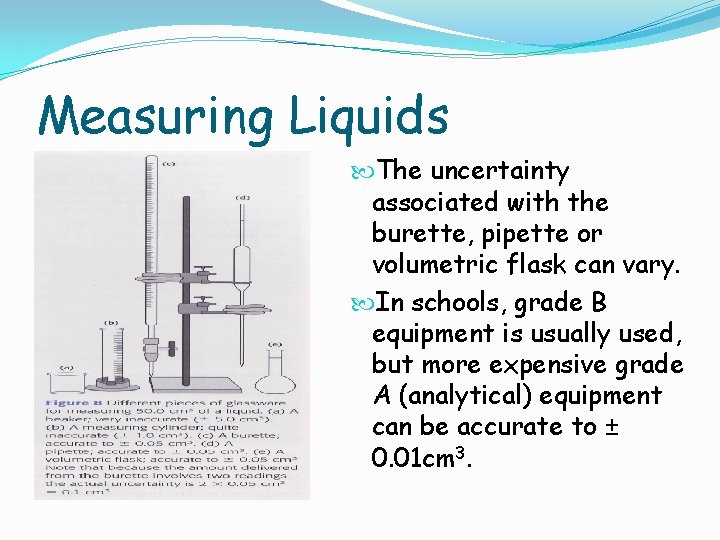
Liquids Pure liquids may be weighed or the volume recorded. The density of the liquid = mass / volume and is usually expressed in g cm-3 or kg dm-3 In the laboratory, volume is usually measured using different apparatus depending on how precisely the volume is required For very approximate volumes a beaker or conical flask can be used. Measuring cylinders are more precise, but still have a large amount of uncertainty. For fixed volumes, volumetric flasks or pipettes are used for precise measurements, and for variable volumes a burette or graduated pipette is used.

Measuring Liquids The uncertainty associated with the burette, pipette or volumetric flask can vary. In schools, grade B equipment is usually used, but more expensive grade A (analytical) equipment can be accurate to ± 0. 01 cm 3.

Solutions Volume is usually used for solutions. 1. 000 litre = 1. 000 dm 3 = 1000 cm 3 Concentration is the amount of solute (dissolved substance) in a known volume of solution (solute plus solvent). It is expressed either in g dm-3 or more usually in mol dm-3 We will work out how to calculate concentrations and carry out volumetric calculations later in the topic.

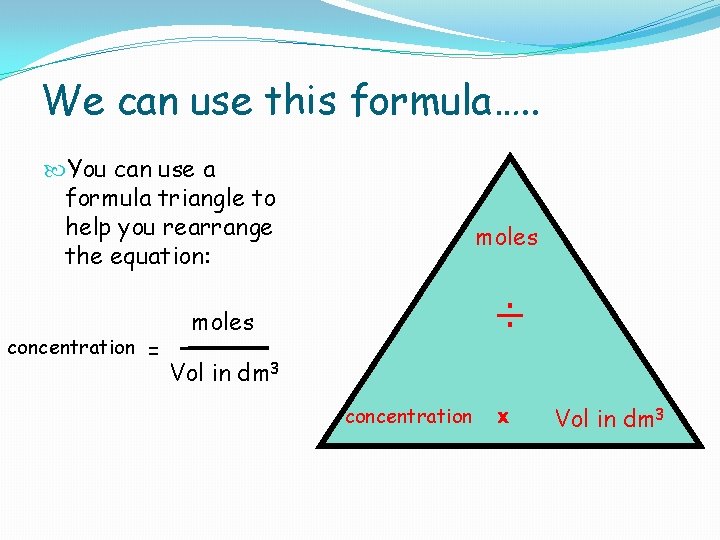
We can use this formula…. . You can use a formula triangle to help you rearrange the equation: concentration = moles ÷ moles Vol in dm 3 concentration x Vol in dm 3

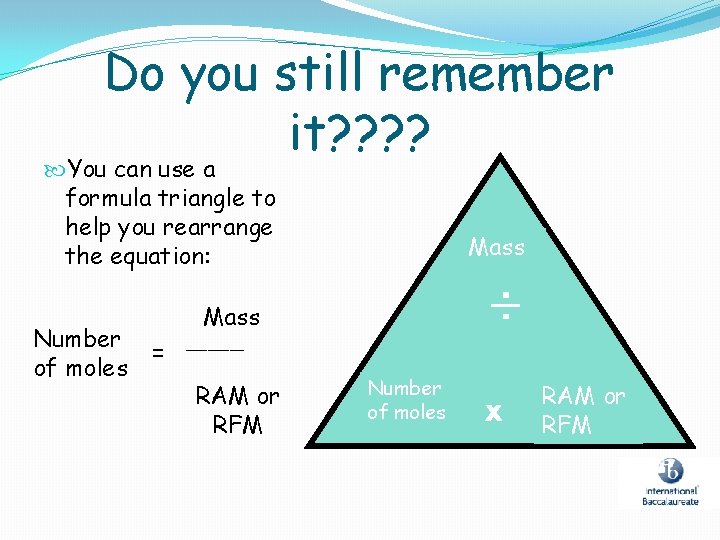
Do you still remember it? ? You can use a formula triangle to help you rearrange the equation: Number of moles Mass ÷ Mass = ____ RAM or RFM Number of moles x RAM or RFM

Concentrations of solutions Concentrations can be measured in: g dm-3 mol dm-3 And you have to be able to convert between them You have already practiced converting moles into grams and vice versa. When you are doing the conversions in concentration sums, the only thing that is different is the amount of substance you are talking about happens to be dissolved in 1 dm 3 of solution. This does not affect the sum in any way.

Calculations involving solutions A solution of sodium hydroxide, Na. OH, had a concentration of 4 g dm-3. What is its concentration in mol dm-3? (H = 1; O = 16; Na = 23) 1 mole of Na. OH weighs 40 g 4 g is 4/40 moles = 0. 1 mol 4 g dm-3 is therefore 0. 1 mol dm-3

Calculations involving solutions What is the concentration of a 0. 0500 mol dm-3 solution of sodium carbonate, Na 2 CO 3, in g dm-3? (C = 12; O = 16; Na = 23) 1 mol Na 2 CO 3 weighs 106 g 0. 0500 mol weighs 0. 0500 x 106 g = 5. 30 g So 0. 0500 mol dm-3 is therefore 5. 30 g dm-3

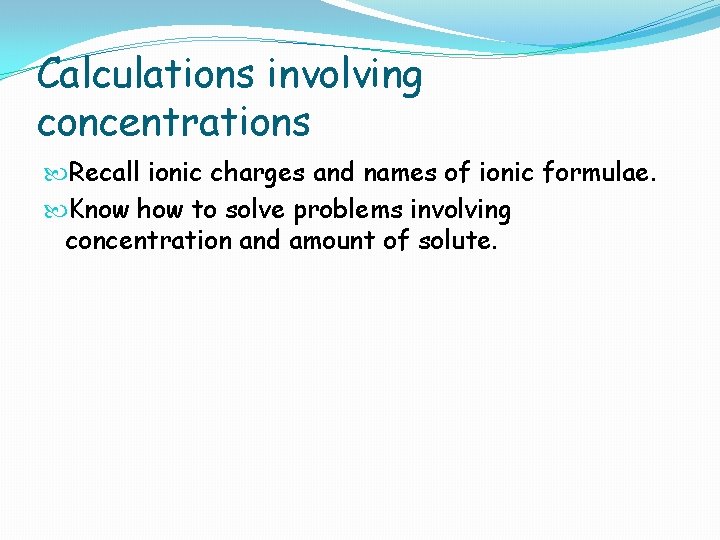
Calculations involving concentrations Recall ionic charges and names of ionic formulae. Know how to solve problems involving concentration and amount of solute.

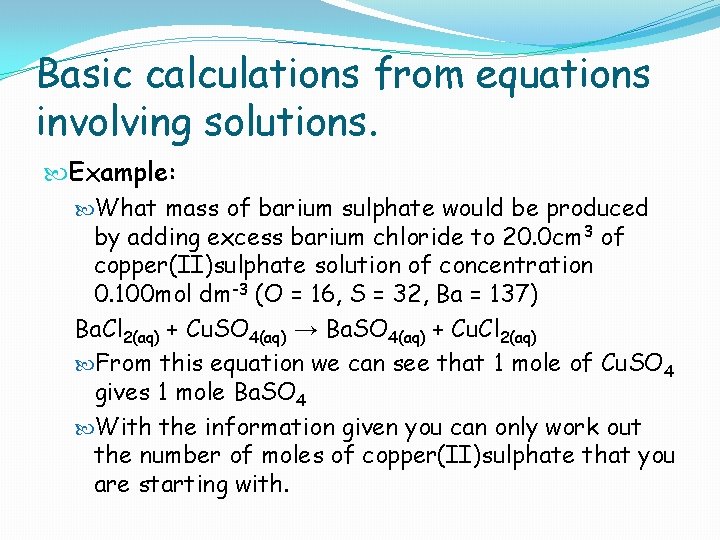
Basic calculations from equations involving solutions. Example: What mass of barium sulphate would be produced by adding excess barium chloride to 20. 0 cm 3 of copper(II)sulphate solution of concentration 0. 100 mol dm-3 (O = 16, S = 32, Ba = 137) Ba. Cl 2(aq) + Cu. SO 4(aq) → Ba. SO 4(aq) + Cu. Cl 2(aq) From this equation we can see that 1 mole of Cu. SO 4 gives 1 mole Ba. SO 4 With the information given you can only work out the number of moles of copper(II)sulphate that you are starting with.

Volume of Cu. SO 4 in Qn 20 Number of moles of = -----Cu. SO 4 1000 --- x 0. 100 Converts cm 3 to dm 3 = 0. 00200 mol Concentration of Cu. SO 4 given in qn.

Going back to the question!! If 1 mole of Cu. SO 4 gives 1 mole of Ba. SO 4 therefore 0. 00200 mol of Cu. SO 4 will give 0. 00200 mol of Ba. SO 4 RFM of 1 mol of Ba. SO 4 = 233 Therefore 0. 00200 mol of Ba. SO 4 = 0. 00200 x = 0. 466 g of Ba. SO 4 233

Next example: What is the maximum mass of calcium carbonate which will react with 25 cm 3 of 2. 00 mol dm-3 hydrochloric acid? (C = 12, O = 16, Ca = 40) Write the balanced symbol equation. Calculate the number of moles of hydrochloric acid. Work out the ratio of moles that HCl reacts with Ca. CO 3 Calculate the number of moles of Calcium chloride Work out the RFM of calcium carbonate What is the maximum mass which could react with HCl?

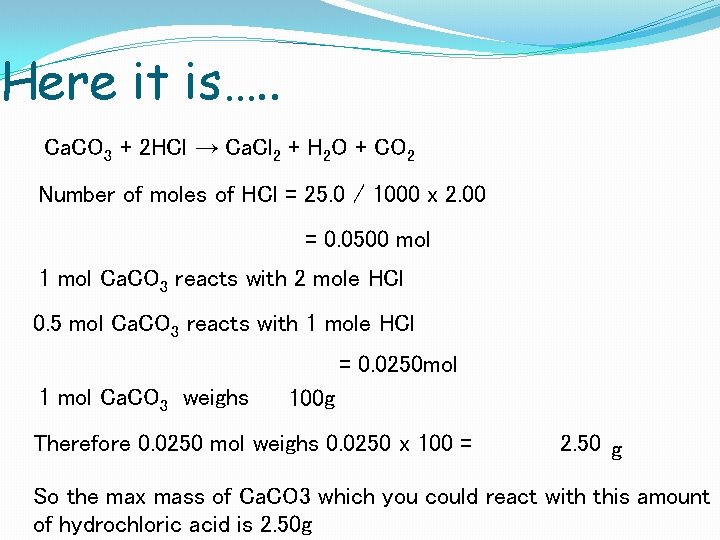
Here it is…. . Ca. CO 3 + 2 HCl → Ca. Cl 2 + H 2 O + CO 2 Number of moles of HCl = 25. 0 / 1000 x 2. 00 = 0. 0500 mol 1 mol Ca. CO 3 reacts with 2 mole HCl = 0. 0250 mol 1 mol Ca. CO 3 weighs 100 g Therefore 0. 0250 mol weighs 0. 0250 x 100 =2. 50 g So the max mass of Ca. CO 3 which you could react with this amount of hydrochloric acid is 2. 50 g

Here it is…. . Ca. CO 3 + 2 HCl → Ca. Cl 2 + H 2 O + CO 2 Number of moles of HCl = 25. 0 / 1000 x 2. 00 = 0. 0500 mol 1 mol Ca. CO 3 reacts with 2 mole HCl 0. 5 mol Ca. CO 3 reacts with 1 mole HCl = 0. 0250 mol 1 mol Ca. CO 3 weighs 100 g Therefore 0. 0250 mol weighs 0. 0250 x 100 = 2. 50 g So the max mass of Ca. CO 3 which you could react with this amount of hydrochloric acid is 2. 50 g

Last one…. What is the minimum volume of 0. 500 mol dm-3 sulphuric acid needed to react with 0. 240 g of magnesium? (Mg = 24) Write a balanced symbol equation. Work out the ratio which magnesium reacts with sulphuric acid. Calculate the number of moles of magnesium Calculate the number of moles of sulphuric acid Calculate the volume of sulphuric acid needed.

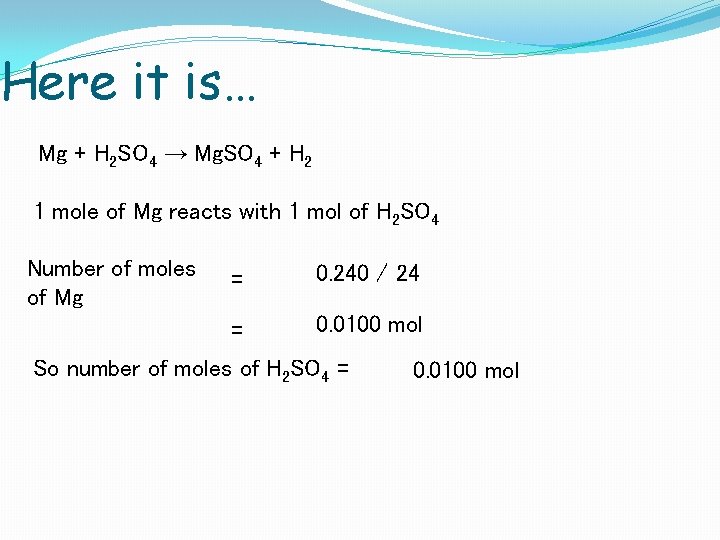
Here it is… Mg + H 2 SO 4 → Mg. SO 4 + H 2 1 mole of Mg reacts with 1 mol of H 2 SO 4 Number of moles of Mg = 0. 240 / 24 = 0. 0100 mol So number of moles of H 2 SO 4 = 0. 0100 mol

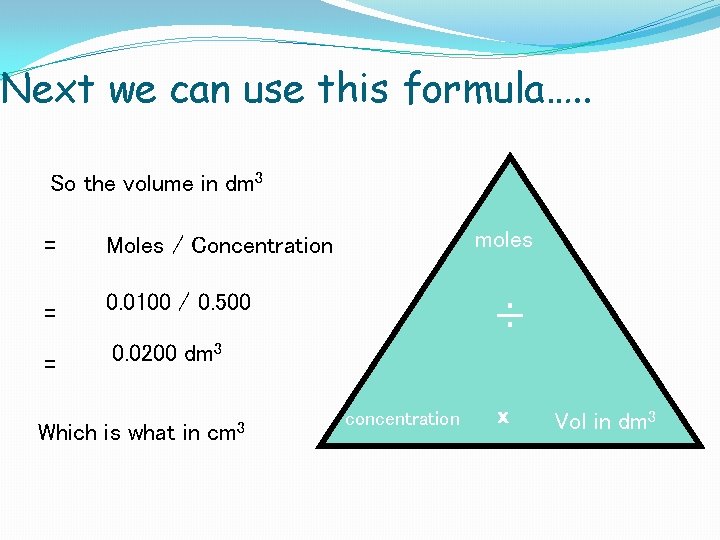
Next we can use this formula…. . So the volume in dm 3 = Moles / Concentration = 0. 0100 / 0. 500 = 0. 0200 dm 3 Which is what in cm 3 moles ÷ concentration x Vol in dm 3

Calculations involving concentrations Know how to solve problems involving concentration and amount of solute. Know how to solve simple titration equations

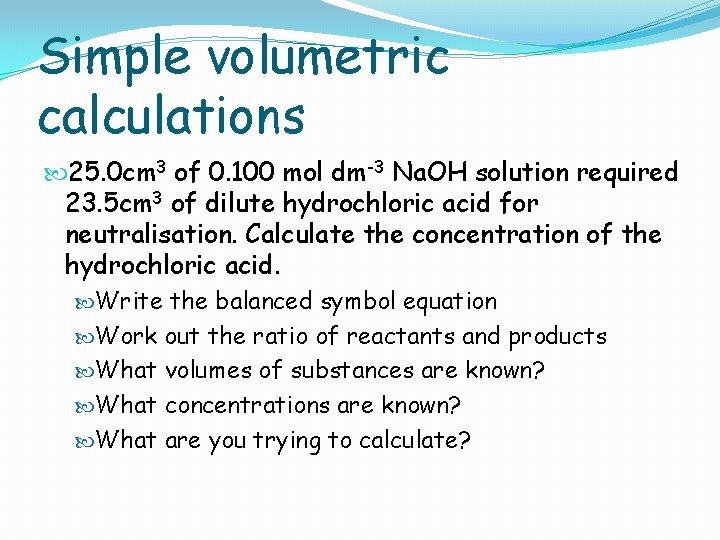
Simple volumetric calculations 25. 0 cm 3 of 0. 100 mol dm-3 Na. OH solution required 23. 5 cm 3 of dilute hydrochloric acid for neutralisation. Calculate the concentration of the hydrochloric acid. Write the balanced symbol equation Work out the ratio of reactants and products What volumes of substances are known? What concentrations are known? What are you trying to calculate?

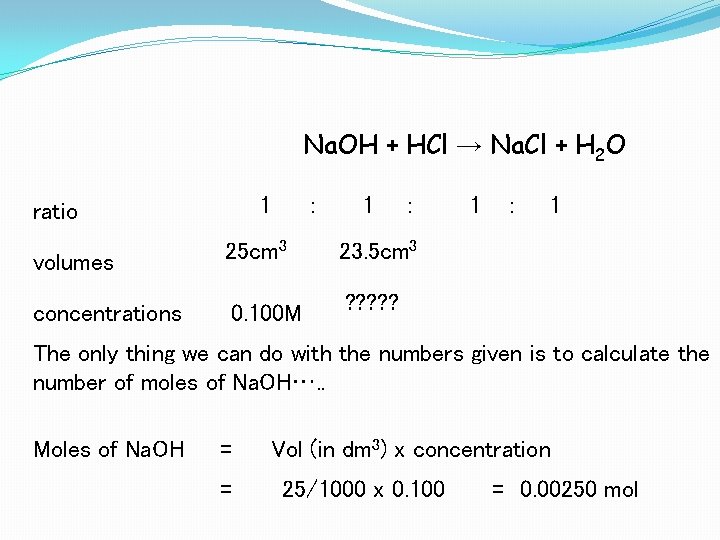
Na. OH + HCl → Na. Cl + H 2 O 1 ratio : 1 : volumes 25 cm 3 23. 5 cm 3 concentrations 0. 100 M ? ? ? 1 : 1 The only thing we can do with the numbers given is to calculate the number of moles of Na. OH…. . Moles of Na. OH = = Vol (in dm 3) x concentration 25/1000 x 0. 100 = 0. 00250 mol

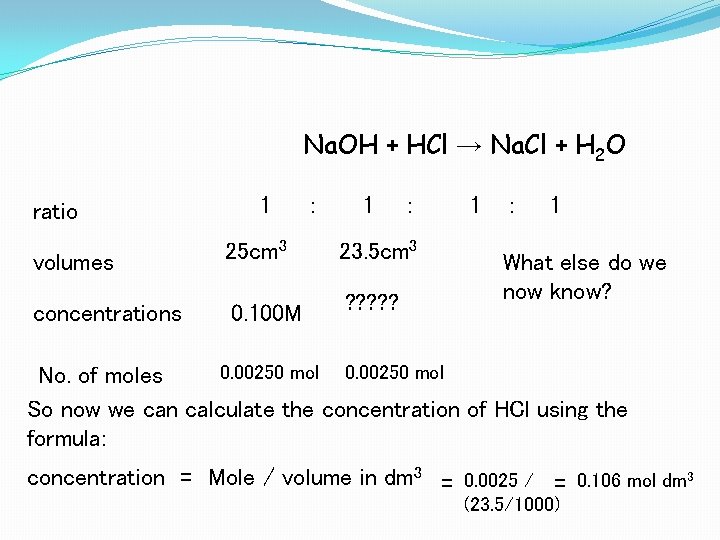
Na. OH + HCl → Na. Cl + H 2 O ratio 1 : volumes 25 cm 3 23. 5 cm 3 concentrations 0. 100 M ? ? ? 1 : 1 What else do we now know? 0. 00250 mol No. of moles So now we can calculate the concentration of HCl using the formula: concentration = Mole / volume in dm 3 = 0. 0025 / = 0. 106 mol dm 3 (23. 5/1000)

Mass and Gaseous volume relationships Calculate theoretical yields from chemical equations. Determine the limiting reactant and the reactant in excess when quantities of reacting substances are given Solve problems involving theoretical, experimental and percentage yield.

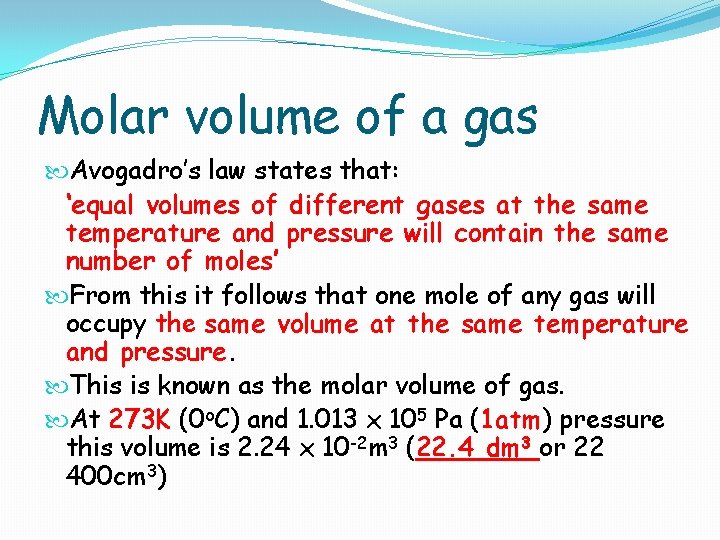
Molar volume of a gas Avogadro’s law states that: ‘equal volumes of different gases at the same temperature and pressure will contain the same number of moles’ From this it follows that one mole of any gas will occupy the same volume at the same temperature and pressure. This is known as the molar volume of gas. At 273 K (0 o. C) and 1. 013 x 105 Pa (1 atm) pressure this volume is 2. 24 x 10 -2 m 3 (22. 4 dm 3 or 22 400 cm 3)

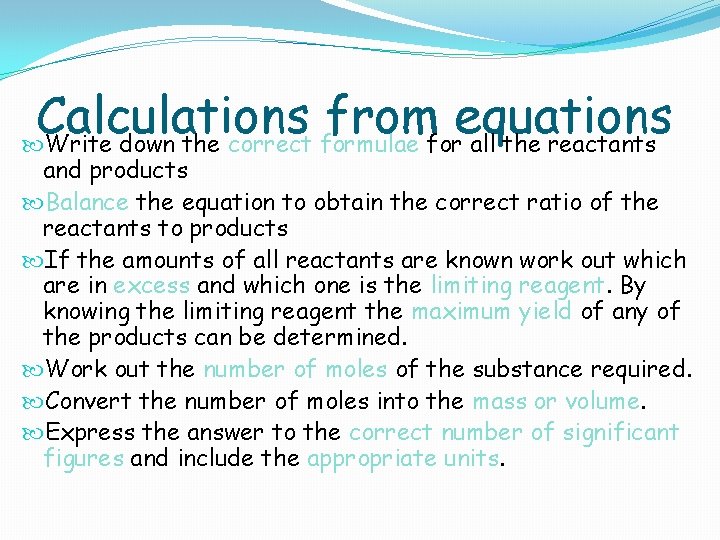
Calculations from equations Write down the correct formulae for all the reactants and products Balance the equation to obtain the correct ratio of the reactants to products If the amounts of all reactants are known work out which are in excess and which one is the limiting reagent. By knowing the limiting reagent the maximum yield of any of the products can be determined. Work out the number of moles of the substance required. Convert the number of moles into the mass or volume. Express the answer to the correct number of significant figures and include the appropriate units.

Example Calculate the volume of hydrogen gas evolved at 273 K and 1 atm pressure when 0. 623 g of magnesium reacts with 27. 3 cm 3 of 1. 25 moldm-3 hydrochloric acid.

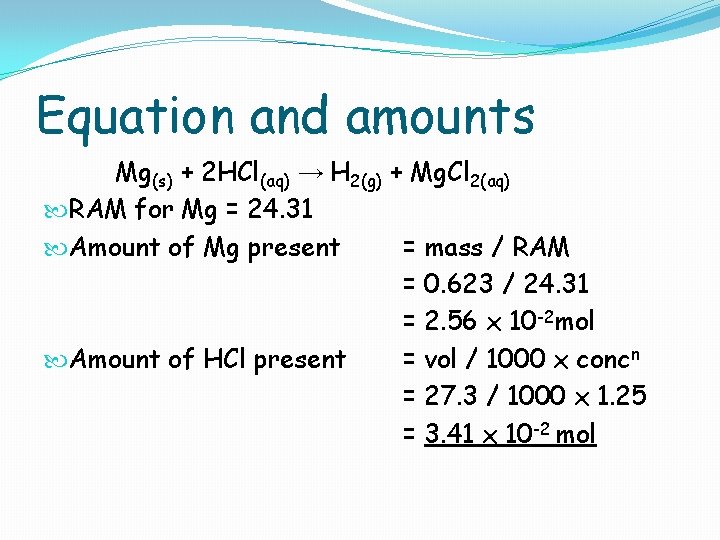
Equation and amounts Mg(s) + 2 HCl(aq) → H 2(g) + Mg. Cl 2(aq) RAM for Mg = 24. 31 Amount of Mg present = mass / RAM = 0. 623 / 24. 31 = 2. 56 x 10 -2 mol Amount of HCl present = vol / 1000 x concn = 27. 3 / 1000 x 1. 25 = 3. 41 x 10 -2 mol

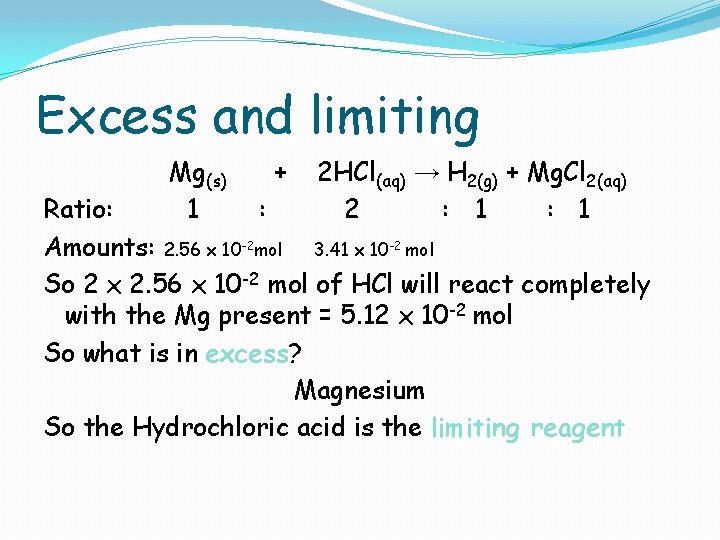
Excess and limiting Mg(s) 1 + 2 HCl(aq) → H 2(g) + Mg. Cl 2(aq) 2 : 1 Ratio: : Amounts: 2. 56 x 10 -2 mol 3. 41 x 10 -2 mol So 2 x 2. 56 x 10 -2 mol of HCl will react completely with the Mg present = 5. 12 x 10 -2 mol So what is in excess? Magnesium So the Hydrochloric acid is the limiting reagent

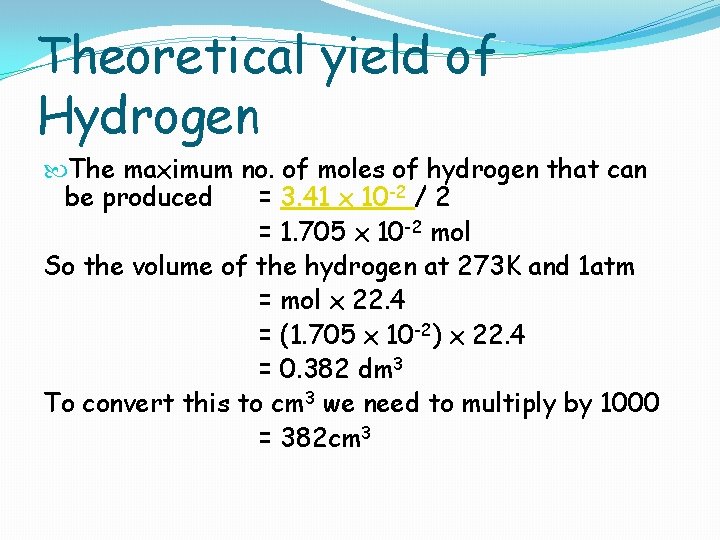
Theoretical yield of Hydrogen The maximum no. of moles of hydrogen that can be produced = 3. 41 x 10 -2 / 2 = 1. 705 x 10 -2 mol So the volume of the hydrogen at 273 K and 1 atm = mol x 22. 4 = (1. 705 x 10 -2) x 22. 4 = 0. 382 dm 3 To convert this to cm 3 we need to multiply by 1000 = 382 cm 3

Gases Mass or volume may be used for gases. Normally it is easier to measure the volume of a gas. However, as well as the amount of gas present, the volume of a gas also depends on the pressure and the temperature. The physical behaviour of all gases is governed by the ideal gas equation: where: p represents the pressure. PV=n. RT V represents the volume measured in m 3 N represents the amount of gas in moles = mass of gas/ Mr T represents the absolute temperature measured in kelvin K. R represents the gas constant.

Gases The units of the gas constant R can be derived from the equation. p x V = N m-2 x m 3 n x T = mol K So the SI units of R must be J K-1 mol R has the value 8. 314 J K constants in science. -1 = J -1 mol -1, and is one of the best known

Ideal Gases The gas equation p. V = n. RT is only true for an ideal gas. Unlike ideal gases, real gases such as oxygen or hydrogen do not obey the law equation exactly. This is because there are still some weak attractive forces between the molecules in the gas, and the molecules themselves occupy some space even though most of the volume of a gas is empty space. However, for practical purposes we can use this ideal gas equation to describe the behaviour of real gases.

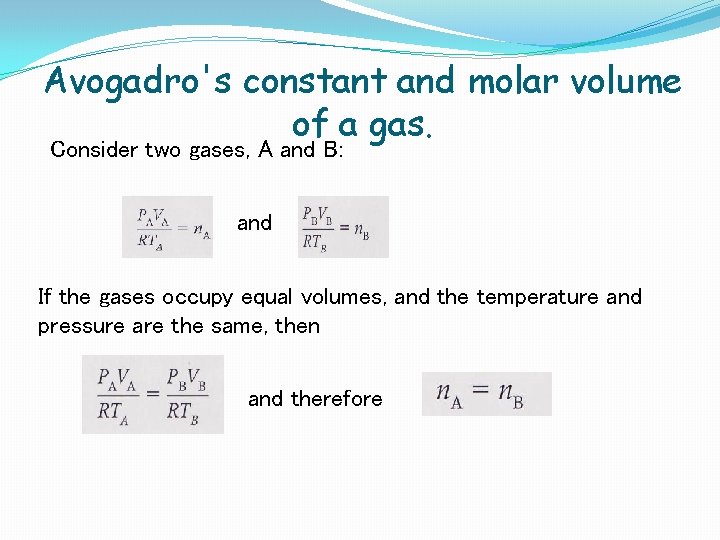
Avogadro's constant and molar volume of a gas. Consider two gases, A and B: and If the gases occupy equal volumes, and the temperature and pressure are the same, then and therefore

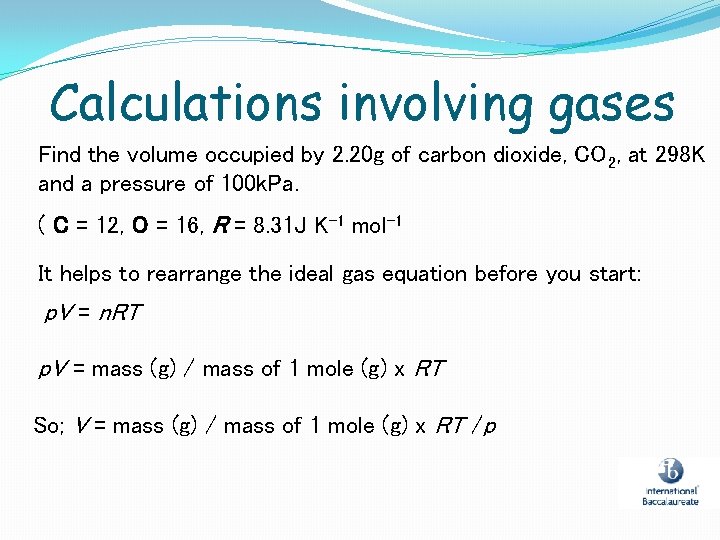
Calculations involving gases Find the volume occupied by 2. 20 g of carbon dioxide, CO 2, at 298 K and a pressure of 100 k. Pa. ( C = 12, O = 16, R = 8. 31 J K-1 mol-1 It helps to rearrange the ideal gas equation before you start: p. V = n. RT p. V = mass (g) / mass of 1 mole (g) x RT So; V = mass (g) / mass of 1 mole (g) x RT /p

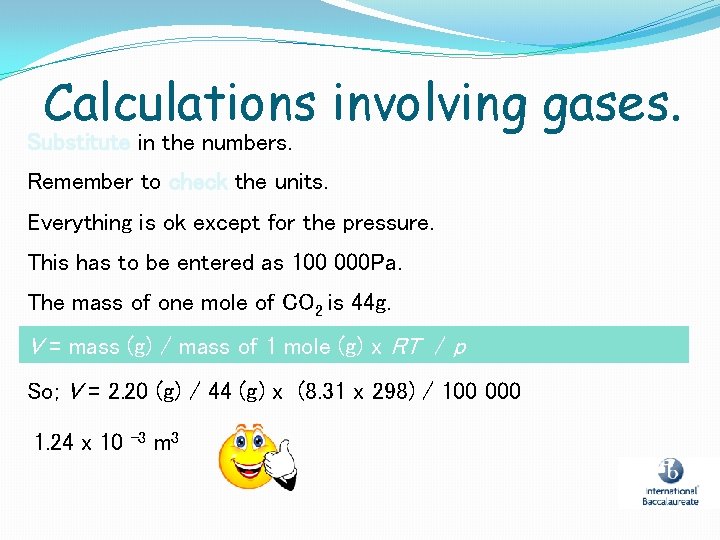
Calculations involving gases. Substitute in the numbers. Remember to check the units. Everything is ok except for the pressure. This has to be entered as 100 000 Pa. The mass of one mole of CO 2 is 44 g. V = mass (g) / mass of 1 mole (g) x RT / p So; V = 2. 20 (g) / 44 (g) x (8. 31 x 298) / 100 000 1. 24 x 10 -3 m 3

Mole Calculations Mole calculations form the basis of many of the calculations that you will meet in IB, they include calculating: number of moles of material in a given mass of that material. mass of material in a given number of moles of that material. concentrations of solutions. volume of a given number of moles of a gas number of moles of gas in a given volume of that gas volume of a given mass of a gas mass of a given volume of gas molar mass of a gas from the mass and the volume data for the gas.
 Definite loop python
Definite loop python Iteratio.n5
Iteratio.n5 One direction figurative language
One direction figurative language Boarding pass advertising
Boarding pass advertising Pixl knowit gcse chemistry quantitative
Pixl knowit gcse chemistry quantitative Quantitative chemistry
Quantitative chemistry Qualitative analysis chemistry igcse
Qualitative analysis chemistry igcse Quantitative chemistry grade 11
Quantitative chemistry grade 11 Quantitative and qualitative in chemistry
Quantitative and qualitative in chemistry Chapter 20 print advertisements worksheet answers
Chapter 20 print advertisements worksheet answers Quantitative determination of amylase activity
Quantitative determination of amylase activity Quantitative elisa laboratory activity
Quantitative elisa laboratory activity Quantitative blood loss worksheet
Quantitative blood loss worksheet Functional groups ib chemistry
Functional groups ib chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Hog hilton
Hog hilton Put out the light then put out the light
Put out the light then put out the light Robert frost poems
Robert frost poems Out of sight out of mind quote
Out of sight out of mind quote Out, out analysis line by line
Out, out analysis line by line Loto safety talk
Loto safety talk Out out frost
Out out frost Lily gulledge
Lily gulledge Matthew 11 msg
Matthew 11 msg Loto
Loto Out, damned spot! out, i say!
Out, damned spot! out, i say! Find out the odd one out
Find out the odd one out Makna out of sight out of mind
Makna out of sight out of mind Log out tag out deutsch
Log out tag out deutsch Activity 1 look out
Activity 1 look out Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Thang điểm glasgow
Thang điểm glasgow Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc
V cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Các số nguyên tố
Các số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Reactants, products, and leftovers
Reactants, products, and leftovers Debye huckel equation
Debye huckel equation Diagram aoa
Diagram aoa Activity 1 introductory activity
Activity 1 introductory activity Activity 2 finding the sequence
Activity 2 finding the sequence Activity 2
Activity 2 Activity 2
Activity 2 Chemistry dimensions 2 worksheet solutions
Chemistry dimensions 2 worksheet solutions Metric conversions dimensional analysis worksheet
Metric conversions dimensional analysis worksheet Writing chemical formulas criss cross method
Writing chemical formulas criss cross method Separating mixtures worksheet
Separating mixtures worksheet Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Apeman atoms
Apeman atoms Anatomy of a wave
Anatomy of a wave Poonams day out
Poonams day out Dem tell me poem
Dem tell me poem Chapter 23 section 1 war breaks out in europe answer key
Chapter 23 section 1 war breaks out in europe answer key Muddy city solution
Muddy city solution True colors
True colors Latitude and longitude activity worksheet
Latitude and longitude activity worksheet Physical activity pyramid worksheet
Physical activity pyramid worksheet Levels of economic activity examples
Levels of economic activity examples Msds activity worksheet answers
Msds activity worksheet answers 4-4 credit cards worksheet answers
4-4 credit cards worksheet answers