Atoms Moles The Mole A Measurement of Matter





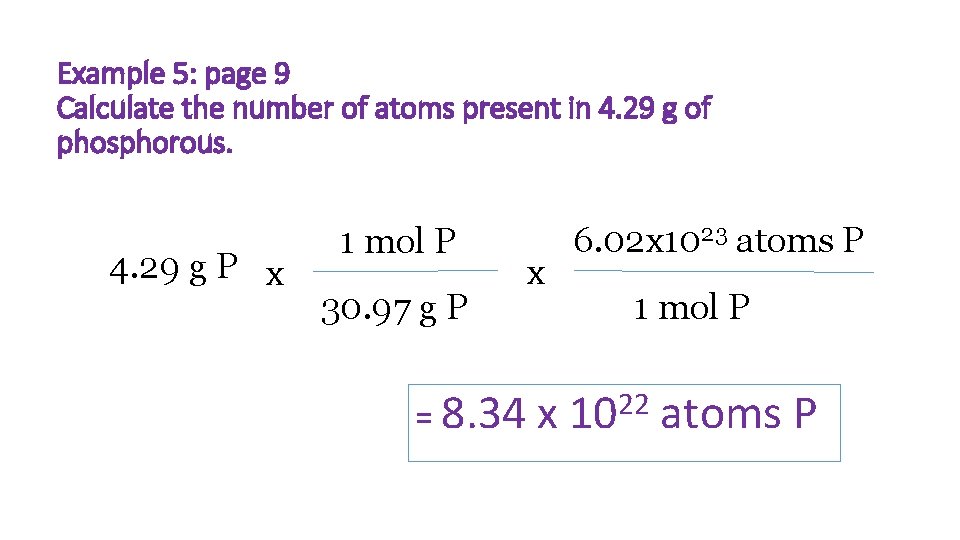


- Slides: 18

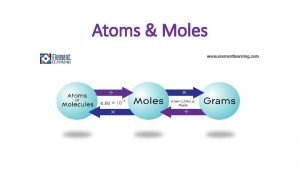
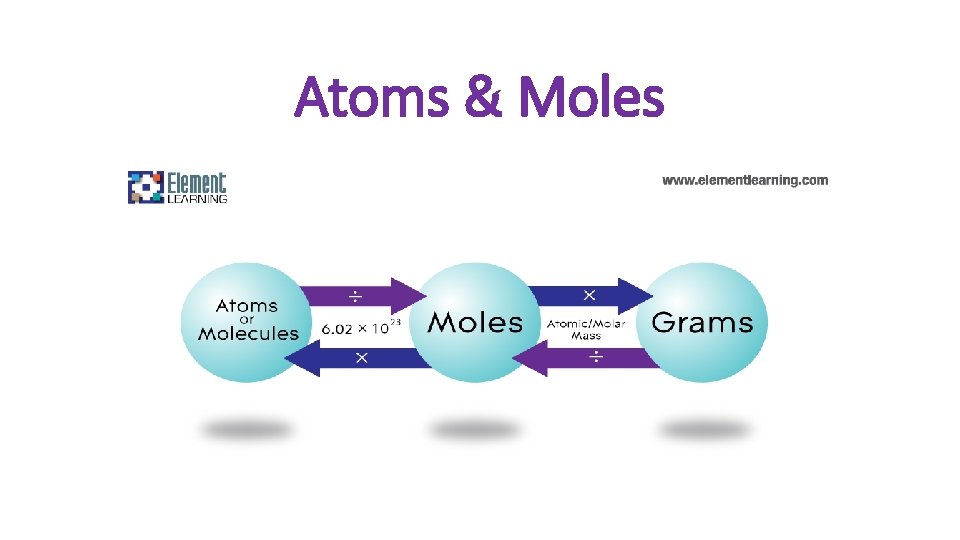
Atoms & Moles

The Mole: A Measurement of Matter • Counting the number of atoms of a substance would very time consuming, because the objects you are counting are so small. • Imagine counting all the grains of sugar you baked into a cake. It would take a really long time! • The mole (mol) is one of the seven base units in the SI system and allows us to easily measures the amount of particles in a substance, even though they are very small. Atoms in garnet, a semi-precious mineral

Avogadro’s Number • 6. 02 x 1023 is called Avogadro’s number. It is named after Amadeo Avogadro who did work in the 1800’s that allowed 6. 02 x 1023 to be calculated and is equal to one mole. • The mole is the “chemist’s dozen”. It is a convenient way to count extremely large numbers of atoms, molecules or ions. 1 mole = 6. 02 x 1023 representative particles

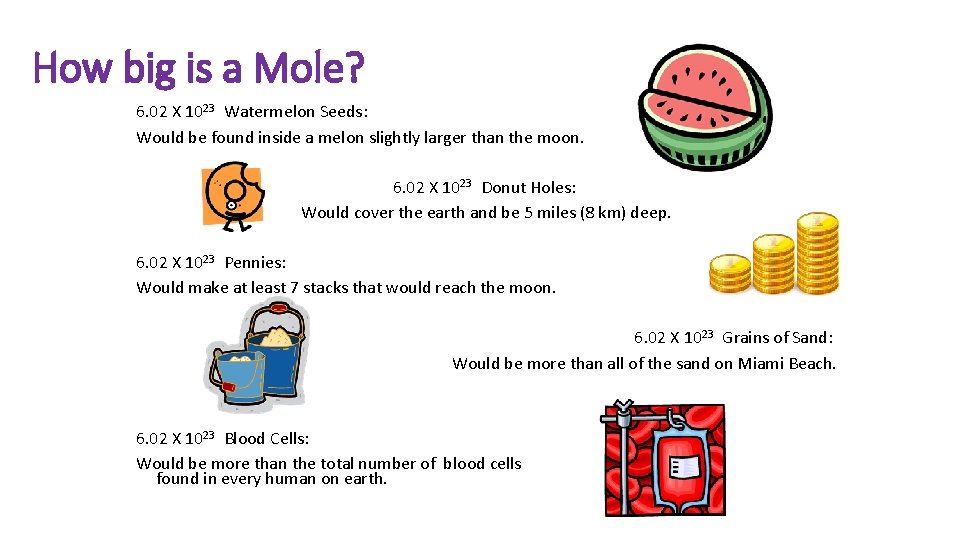
How big is a Mole? 6. 02 X 1023 Watermelon Seeds: Would be found inside a melon slightly larger than the moon. 6. 02 X 1023 Donut Holes: Would cover the earth and be 5 miles (8 km) deep. 6. 02 X 1023 Pennies: Would make at least 7 stacks that would reach the moon. 6. 02 X 1023 Grains of Sand: Would be more than all of the sand on Miami Beach. 6. 02 X 1023 Blood Cells: Would be more than the total number of blood cells found in every human on earth.

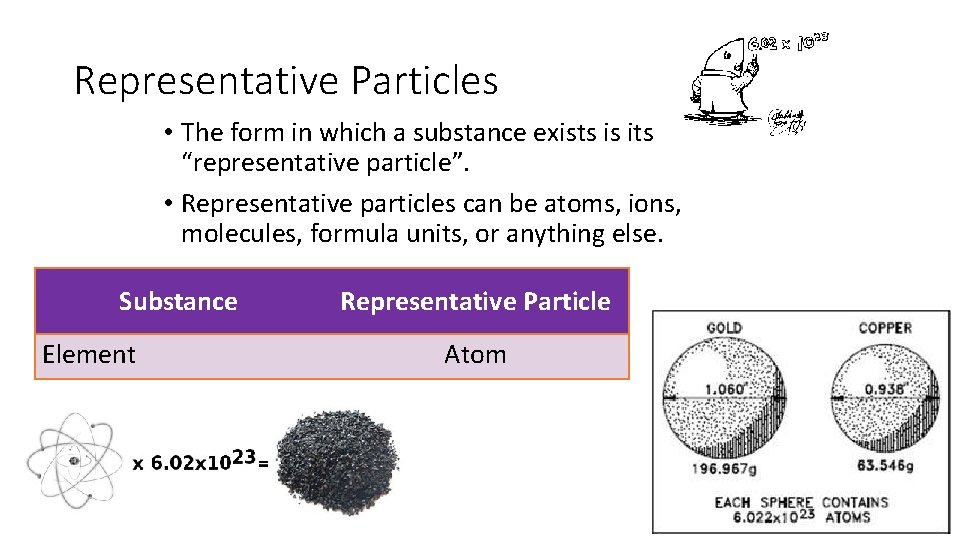
Representative Particles • The form in which a substance exists is its “representative particle”. • Representative particles can be atoms, ions, molecules, formula units, or anything else. Substance Element Representative Particle Atom

Mole-Mass Relationships (A-M-G) Conversion Factors: 1 mol = 6. 02 X 1023

Solving Problems with Avogadro’s Number • We work these problems using dimensional analysis. • 1 mole = 6. 02 x 1023 representative particles • Representative Particles: unit needs to be applicable to what is being calculated.

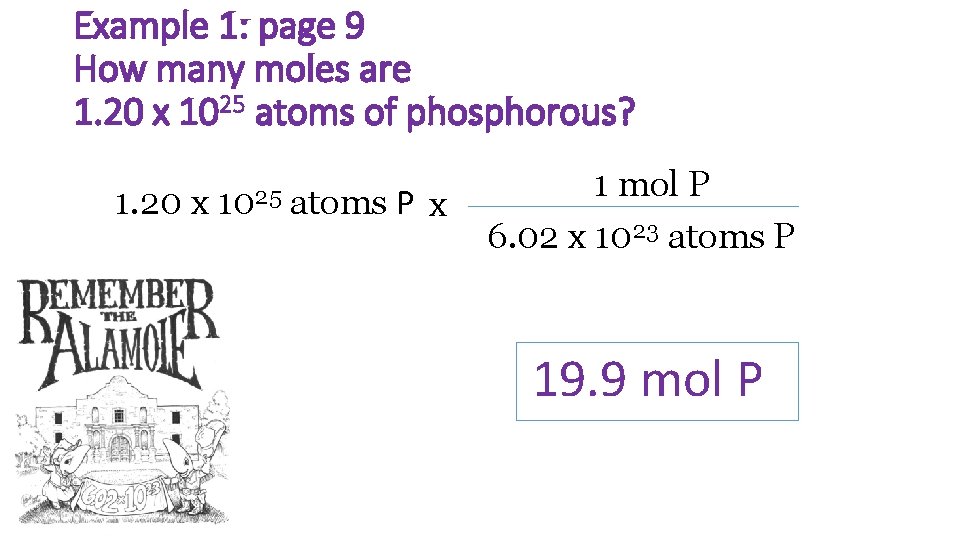
Example 1: page 9 How many moles are 1. 20 x 1025 atoms of phosphorous? 1. 20 x 1025 atoms P x 1 mol P 6. 02 x 1023 atoms P 19. 9 mol P

Example 2: page 9 How many atoms are in 0. 750 mol of Zn? 0. 750 mol Zn x 6. 02 x 1023 atoms Zn 1 mol Zn 4. 52 x 1023 atoms Zn

Mole – Mass Conversions (A-M-G) Conversion factor 1 mol = molar mass (g)

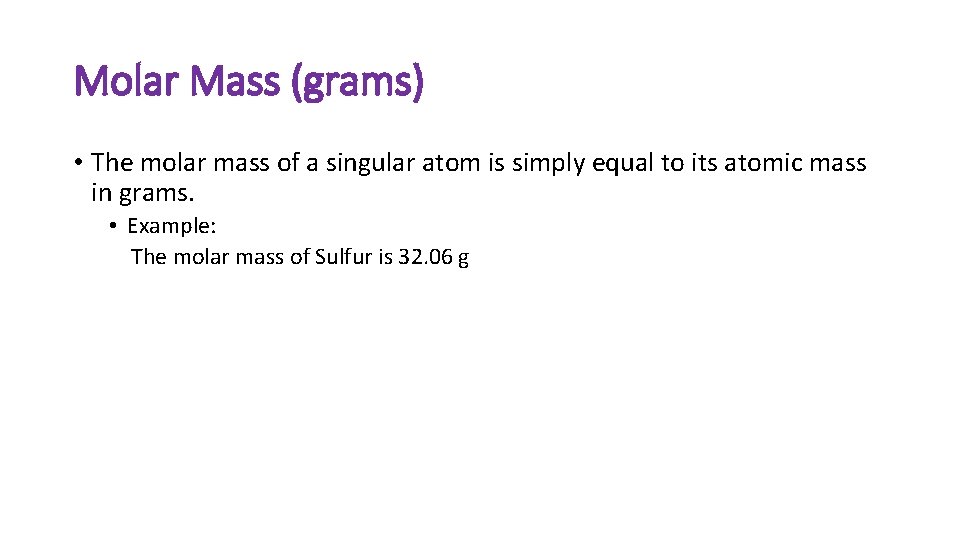
Molar Mass (grams) • The molar mass of a singular atom is simply equal to its atomic mass in grams. • Example: The molar mass of Sulfur is 32. 06 g

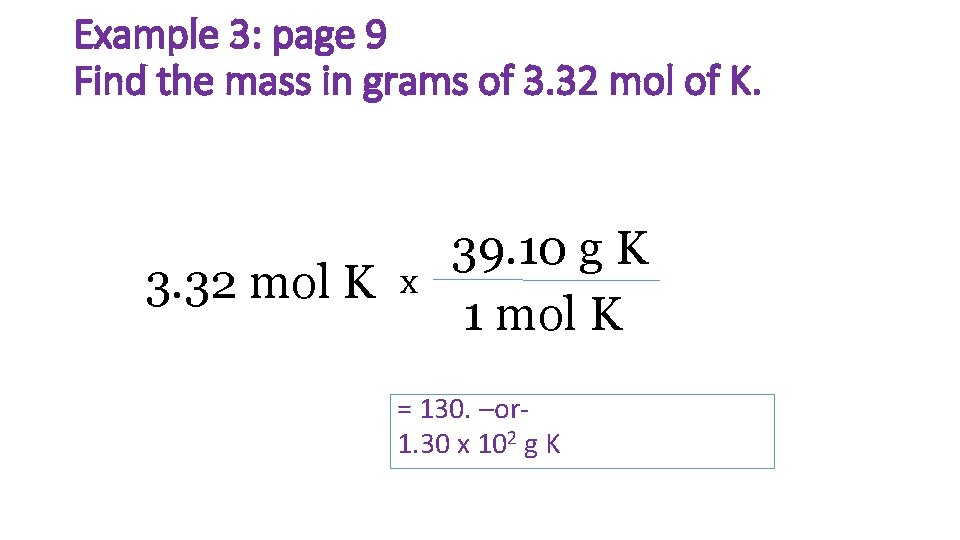
Example 3: page 9 Find the mass in grams of 3. 32 mol of K. 3. 32 mol K x 39. 10 g K 1 mol K = 130. –or- 1. 30 x 102 g K

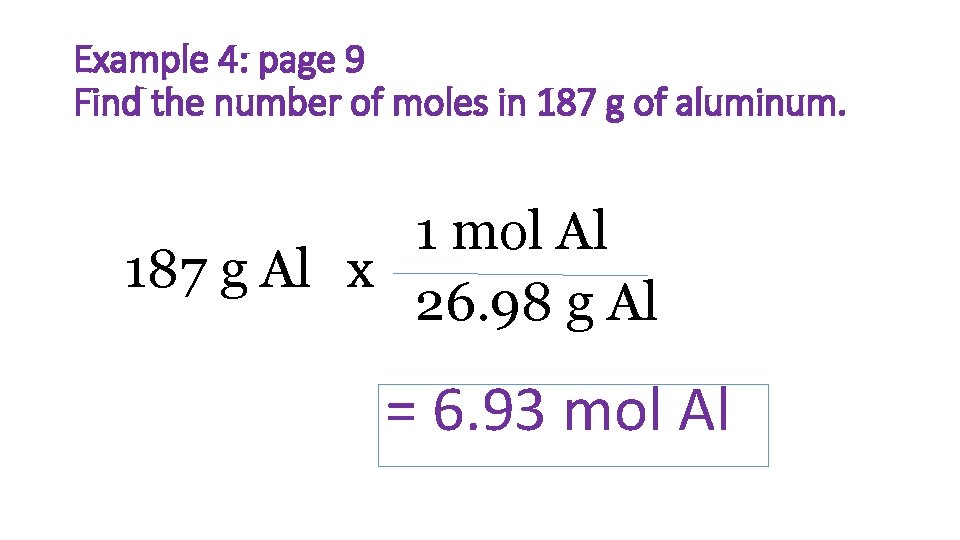
Example 4: page 9 Find the number of moles in 187 g of aluminum. 1 mol Al 187 g Al x 26. 98 g Al = 6. 93 mol Al

Two-Step Mole Problems (A-M-G) Using multiple conversion factors. 1 mol = molar mass 1 mol= 6. 02 X 1023 atoms
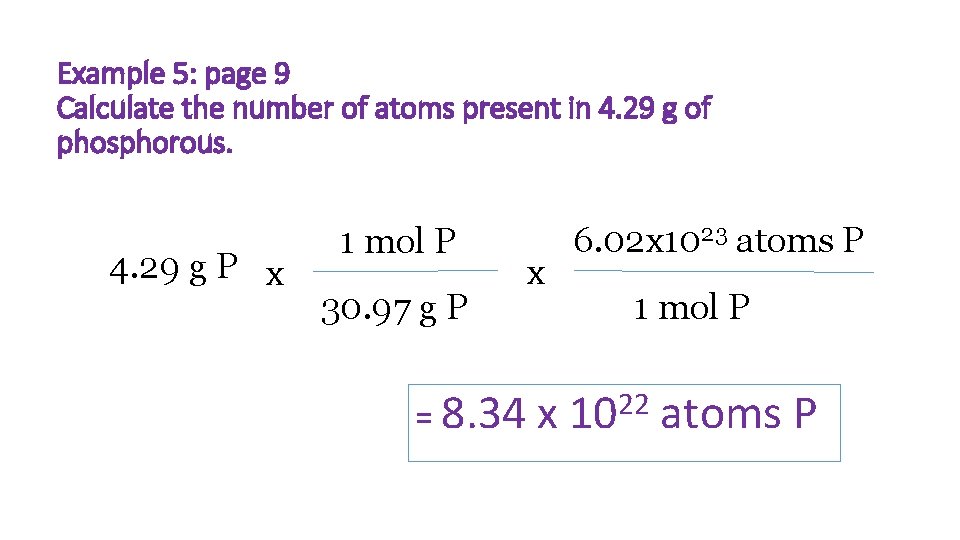
Example 5: page 9 Calculate the number of atoms present in 4. 29 g of phosphorous. 4. 29 g P x 1 mol P 30. 97 g P x 6. 02 x 1023 atoms P 1 mol P 22 = 8. 34 x 10 atoms P

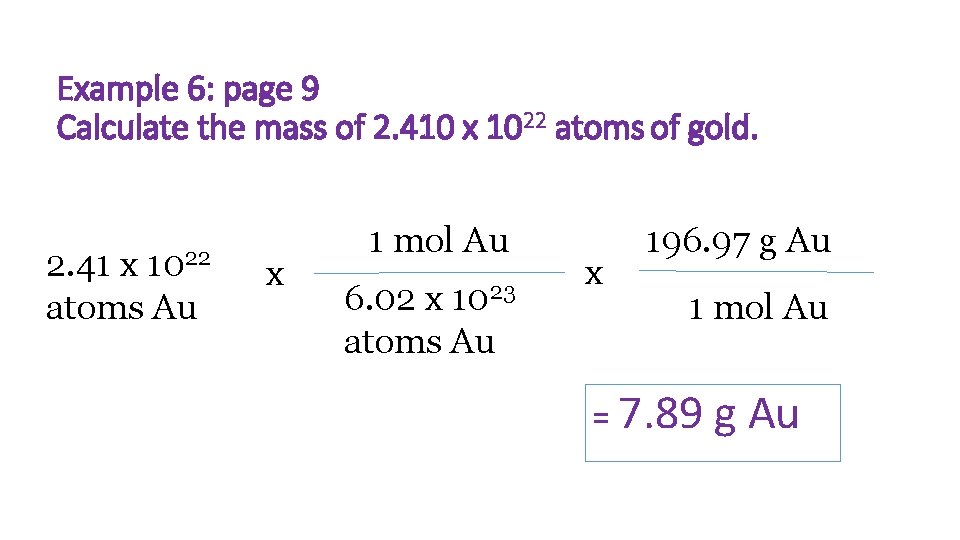
Example 6: page 9 Calculate the mass of 2. 410 x 1022 atoms of gold. 1022 2. 41 x atoms Au x 1 mol Au 6. 02 x 1023 atoms Au x 196. 97 g Au 1 mol Au = 7. 89 g Au

Complete practice problems 1 -5 on bottom of page 9 AND complete page 10 in your packet for homework!

Discuss the Warm-up • Compare the mass of 1 mole of Al with the mass of 1 mole of S. Which is larger? Why do you think that element has a larger mass? • Compare the mass of 1 mole of Fe with the mass of 1 mole of Sn. Which is larger? Why do you think that element has a larger mass? • Look at the rest of your data. What patterns do you observe in the relationship between the mass of one mole of a sample and the element itself? • Which do you think would weigh more, one mole of Gold or one mole of Silver? Explain. • If we had an additional container containing 1 mole of potassium, what do you think would be the mass of the element in the bag? Why do you think this?
 The mole a measurement of matter
The mole a measurement of matter 10.1 the mole: a measurement of matter
10.1 the mole: a measurement of matter 10.1 the mole a measurement of matter
10.1 the mole a measurement of matter Ideal gas law powerpoint
Ideal gas law powerpoint Moles of atoms
Moles of atoms Atoms to moles
Atoms to moles 1 mole = grams
1 mole = grams Atoms to moles
Atoms to moles Amu vs molar mass
Amu vs molar mass Mole map
Mole map Xkcd mole of moles
Xkcd mole of moles At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity Mole-mole factor
Mole-mole factor Mole-mass-volume relationships
Mole-mass-volume relationships Phosphorus + oxygen
Phosphorus + oxygen Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Stoichiometry mole-mole
Stoichiometry mole-mole Gram to gram conversion
Gram to gram conversion Mol from mass
Mol from mass