Electron Configuration Mapping the electrons Electron Configuration n








- Slides: 32

Electron Configuration Mapping the electrons

Electron Configuration n The way electrons are arranged around the nucleus.

Quantum Mechanical Model n n 1920’s Werner Heisenberg (Uncertainty Principle) Louis de Broglie (electron has wave properties) Erwin Schrodinger (mathematical equations using probability, quantum numbers)

Heisenberg uncertainty principle n it is impossible to determine simultaneously both the position and velocity of an electron or any other particle with any great degree of accuracy or certainty.

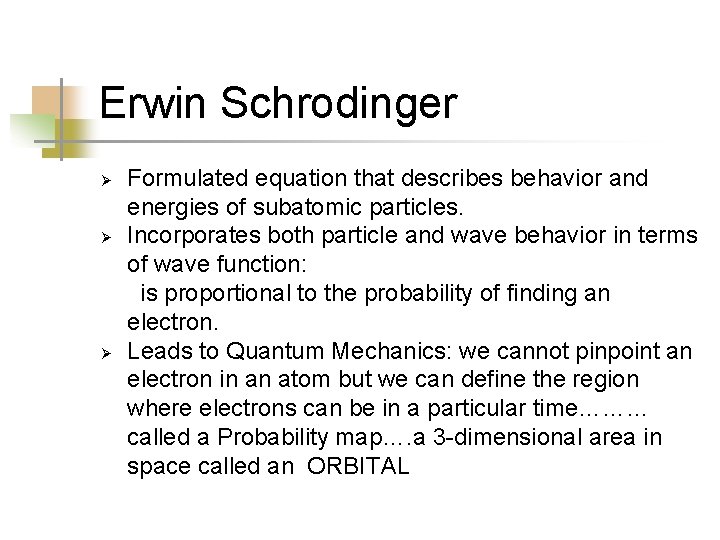
Erwin Schrodinger Ø Ø Ø Formulated equation that describes behavior and energies of subatomic particles. Incorporates both particle and wave behavior in terms of wave function: is proportional to the probability of finding an electron. Leads to Quantum Mechanics: we cannot pinpoint an electron in an atom but we can define the region where electrons can be in a particular time……… called a Probability map…. a 3 -dimensional area in space called an ORBITAL

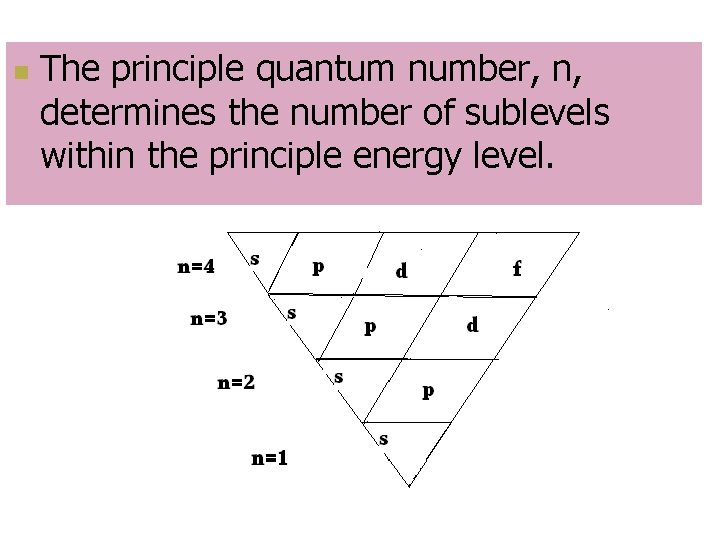
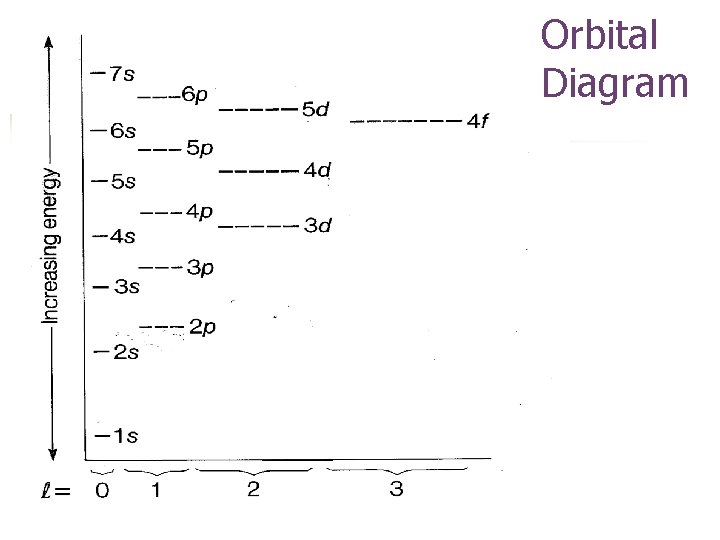
Principal Quantum Number, n n Indicates main energy levels n = 1, 2, 3, 4… n Each main energy level has sub-levels

Energy Sublevels s p d f g

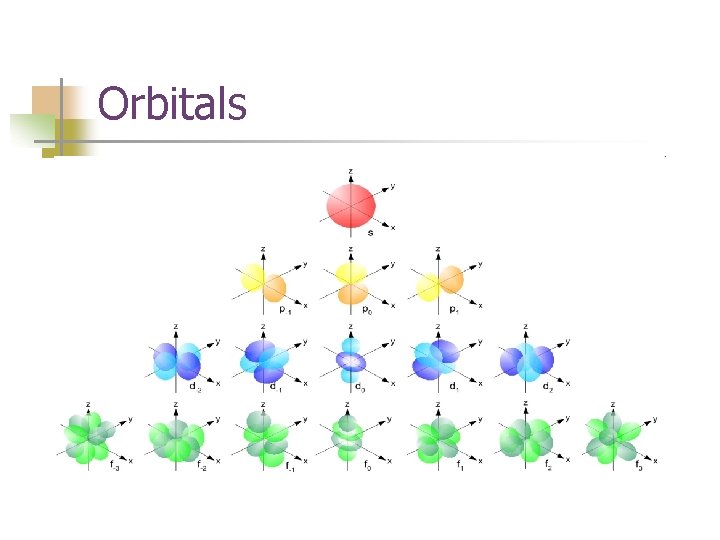
Orbitals

n The principle quantum number, n, determines the number of sublevels within the principle energy level.

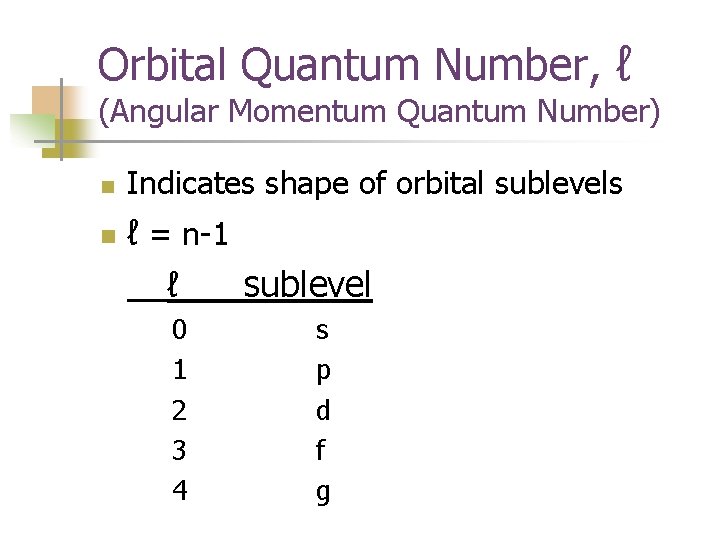
Orbital Quantum Number, ℓ (Angular Momentum Quantum Number) n n Indicates shape of orbital sublevels ℓ = n-1 ℓ sublevel 0 1 2 3 4 s p d f g

Orbital n The space where there is a high probability that it is occupied by a pair of electrons.

Visualizing the orbitals

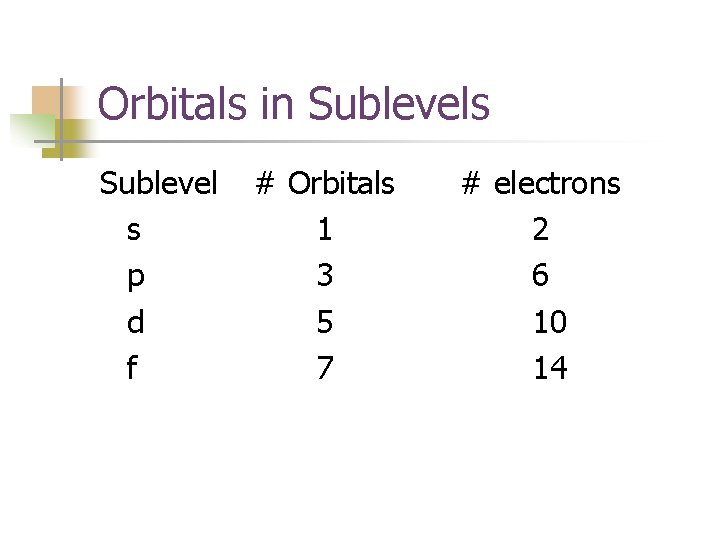
Orbitals in Sublevels Sublevel s p d f # Orbitals 1 3 5 7 # electrons 2 6 10 14

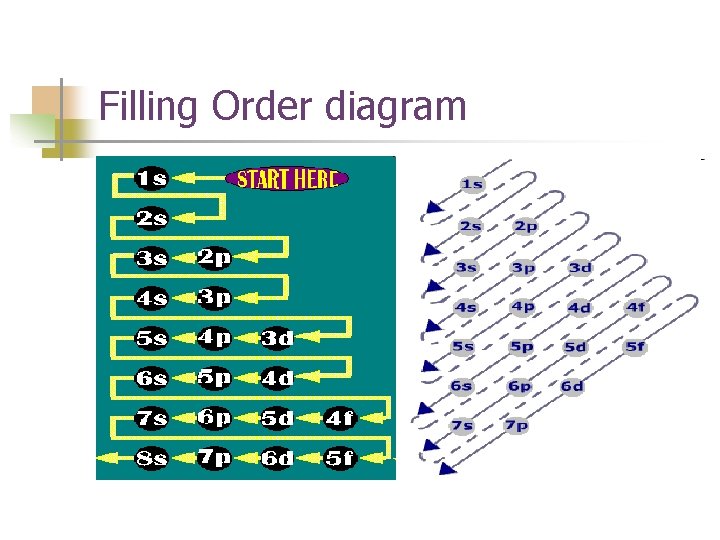
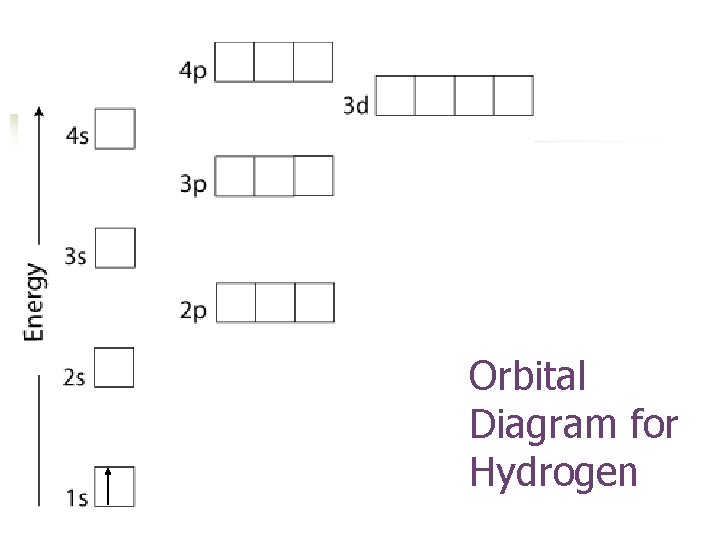
Three rules are used to build the electron configuration: Aufbau principle n Pauli Exclusion Principle n Hund’s Rule n

Aufbau Principle n Electrons occupy orbitals of lower energy first.

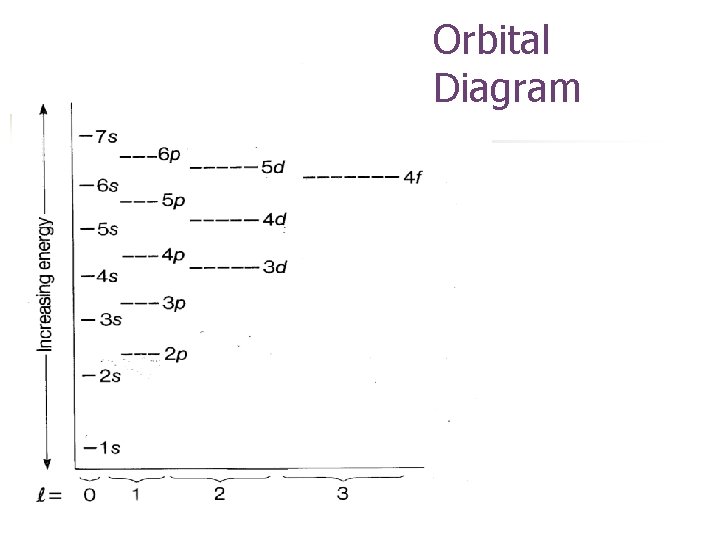
Orbital Diagram

Filling Order diagram

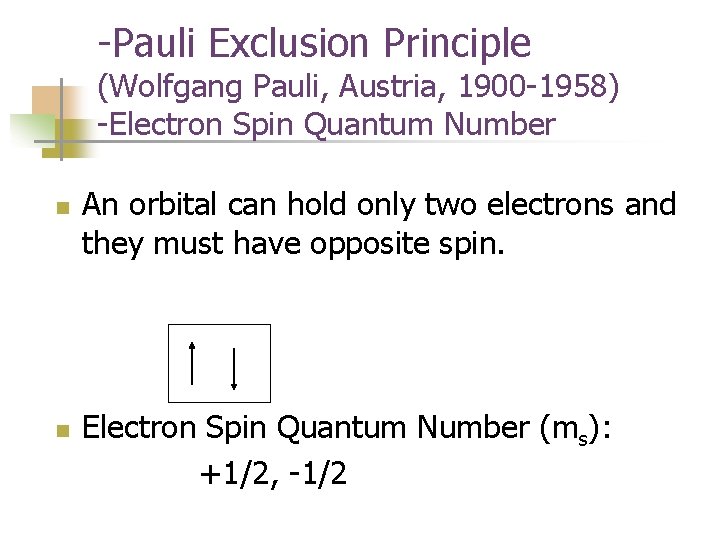
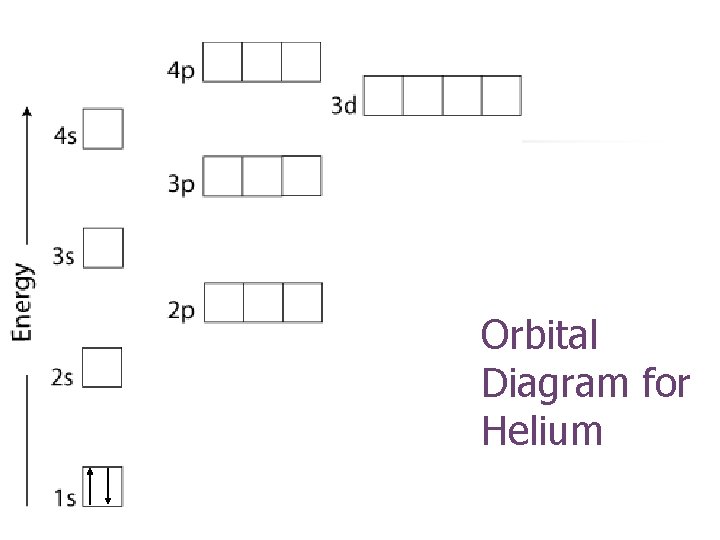
-Pauli Exclusion Principle (Wolfgang Pauli, Austria, 1900 -1958) -Electron Spin Quantum Number n n An orbital can hold only two electrons and they must have opposite spin. Electron Spin Quantum Number (ms): +1/2, -1/2

Hund’s Rule In a set of orbitals, the electrons will fill the orbitals in a way that would give the maximum number of parallel spins (maximum number of unpaired electrons). Analogy: Students could fill each seat of a school bus, one person at a time, before doubling up.

Orbital Diagram for Hydrogen

Orbital Diagram for Helium

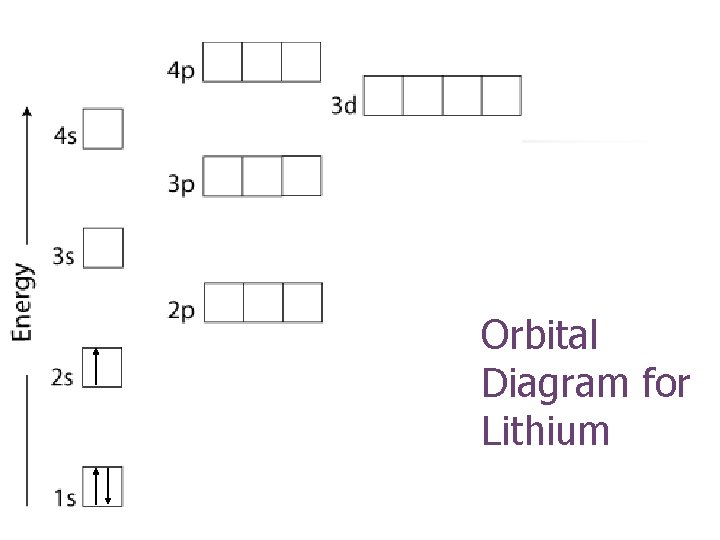
Orbital Diagram for Lithium

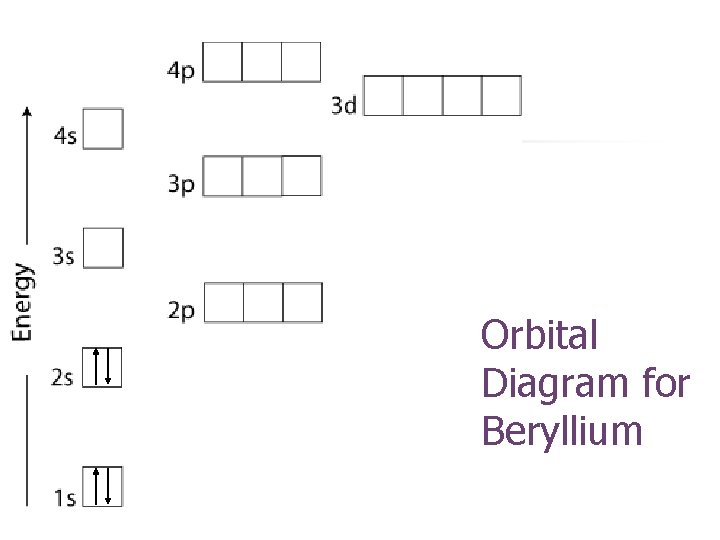
Orbital Diagram for Beryllium

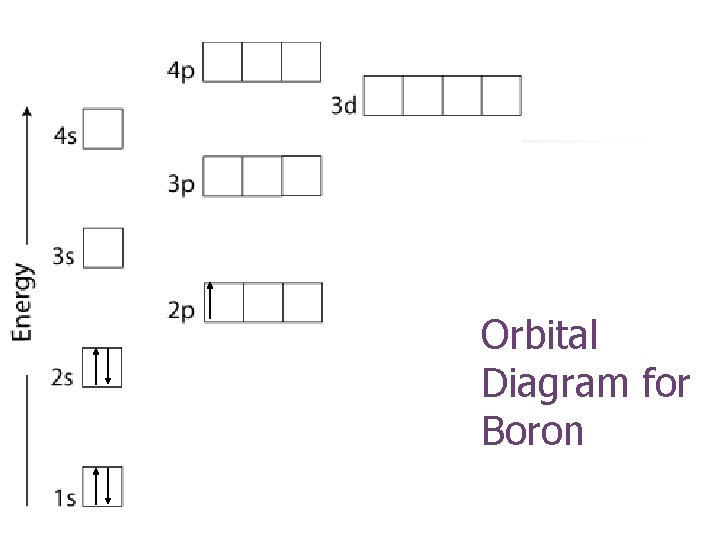
Orbital Diagram for Boron

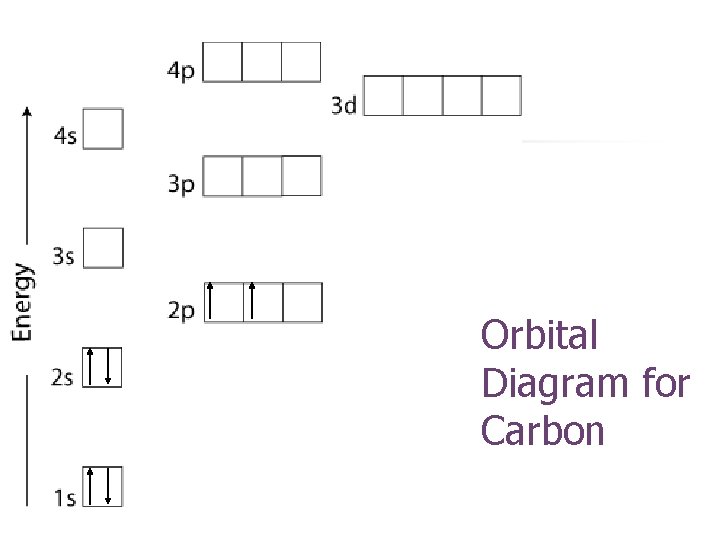
Orbital Diagram for Carbon

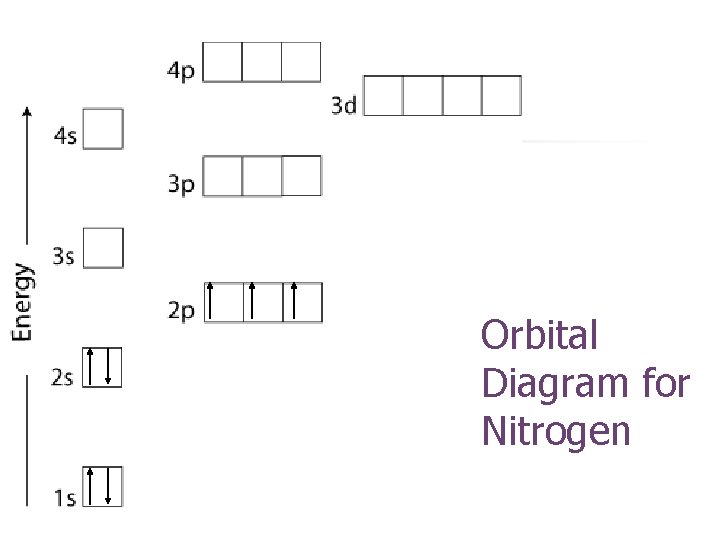
Orbital Diagram for Nitrogen

Orbital Diagram

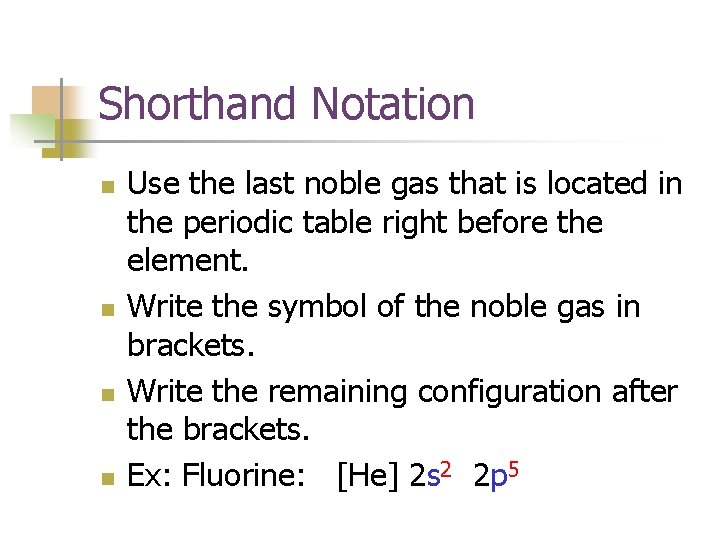
Notations of Electron Configurations n n Standard Shorthand

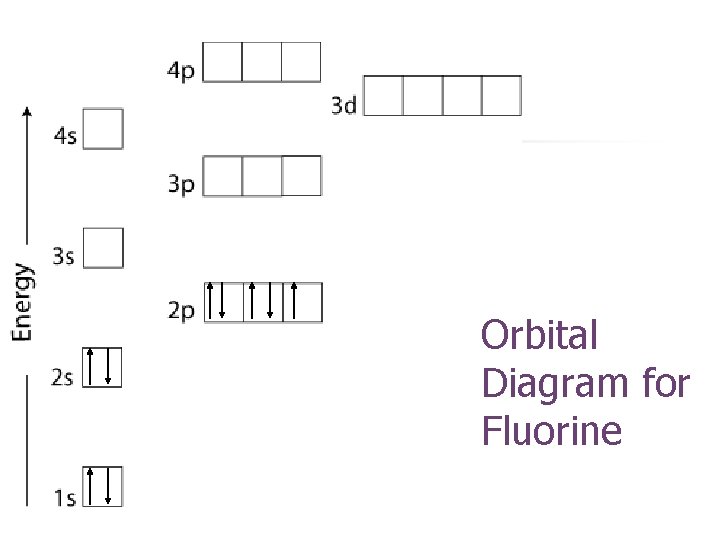
Orbital Diagram for Fluorine

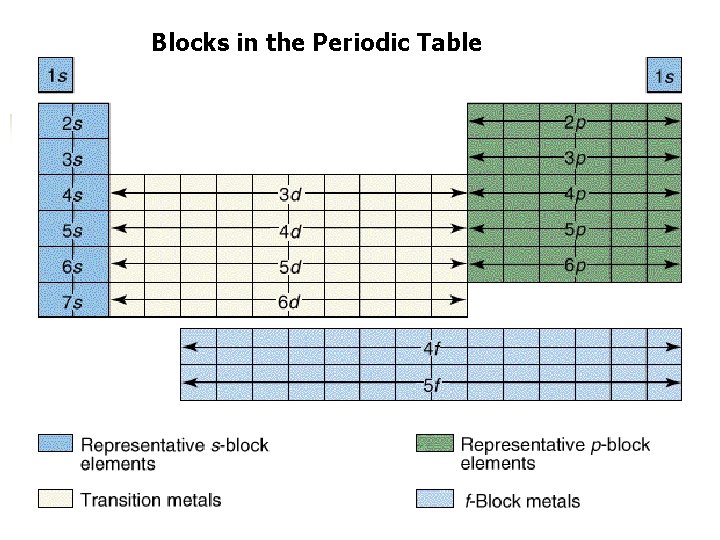
Blocks in the Periodic Table

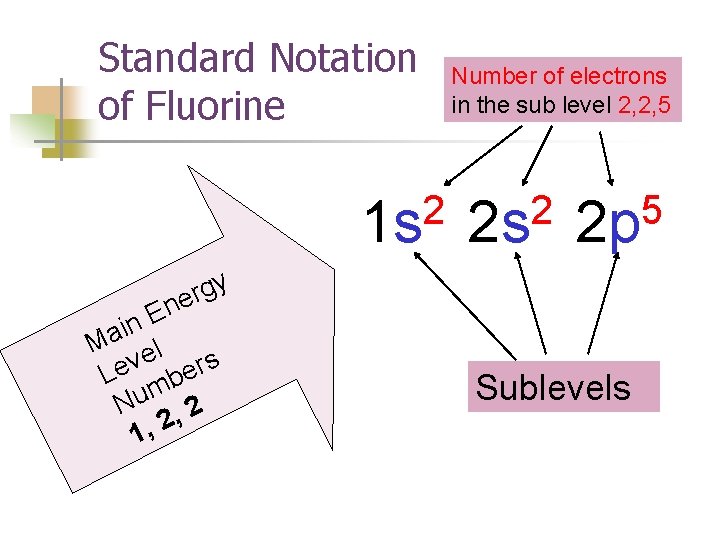
Standard Notation of Fluorine 2 1 s y g r e n E n i Ma el v s r e e L b m Nu , 2 2 , 1 Number of electrons in the sub level 2, 2, 5 2 2 s 5 2 p Sublevels

Shorthand Notation n n Use the last noble gas that is located in the periodic table right before the element. Write the symbol of the noble gas in brackets. Write the remaining configuration after the brackets. Ex: Fluorine: [He] 2 s 2 2 p 5