Ch 4 Electrons in Atoms Electron Configuration Maximum





![Shorthand Configuration Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] 4 Shorthand Configuration Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] 4](https://slidetodoc.com/presentation_image/593ed8f33992ad27cb18762b25a714d7/image-17.jpg)
- Slides: 17

Ch. 4 - Electrons in Atoms Electron Configuration

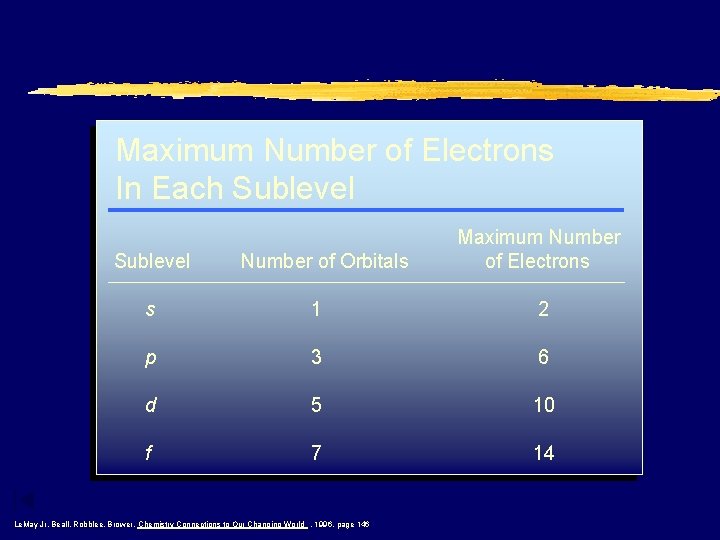
Maximum Number of Electrons In Each Sublevel Number of Orbitals Maximum Number of Electrons s 1 2 p 3 6 d 5 10 f 7 14 Le. May Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 146

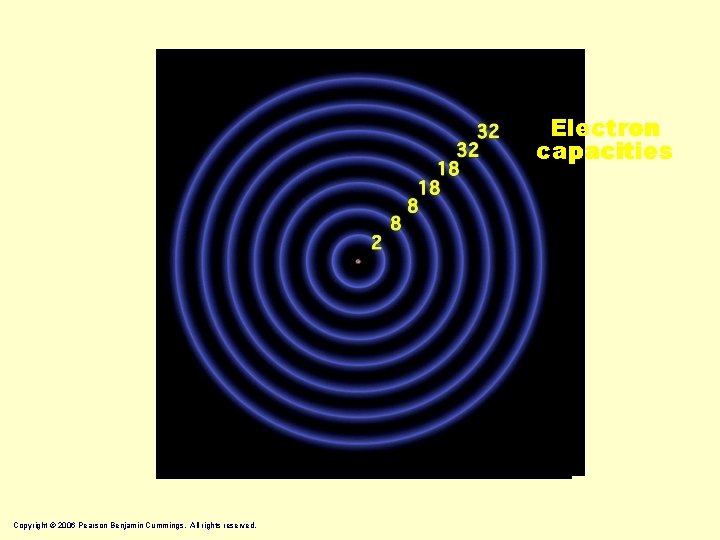
Electron capacities Copyright © 2006 Pearson Benjamin Cummings. All rights reserved.

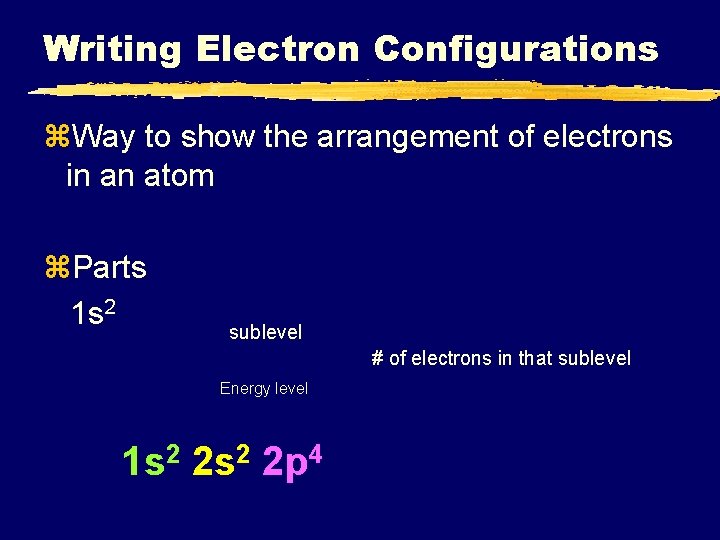
Writing Electron Configurations z. Way to show the arrangement of electrons in an atom z. Parts 1 s 2 sublevel # of electrons in that sublevel Energy level 1 s 2 2 p 4

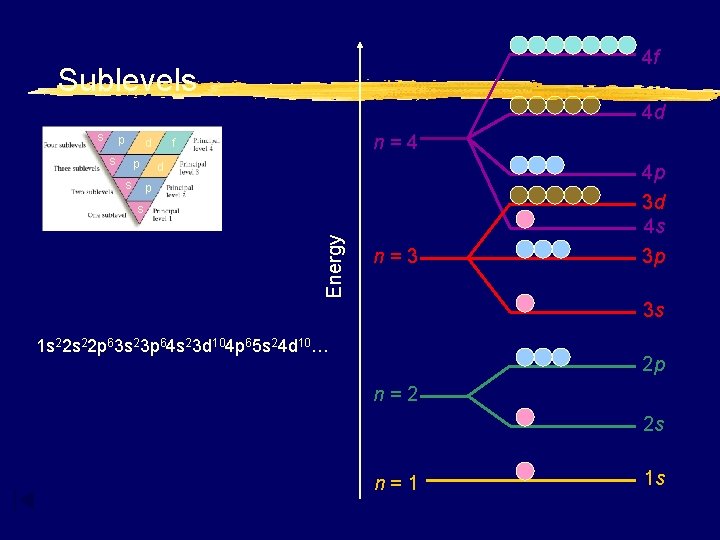
4 f Sublevels 4 d s p s d p s n=4 f d p Energy s n=3 4 p 3 d 4 s 3 p 3 s 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 10… 2 p n=2 2 s n=1 1 s

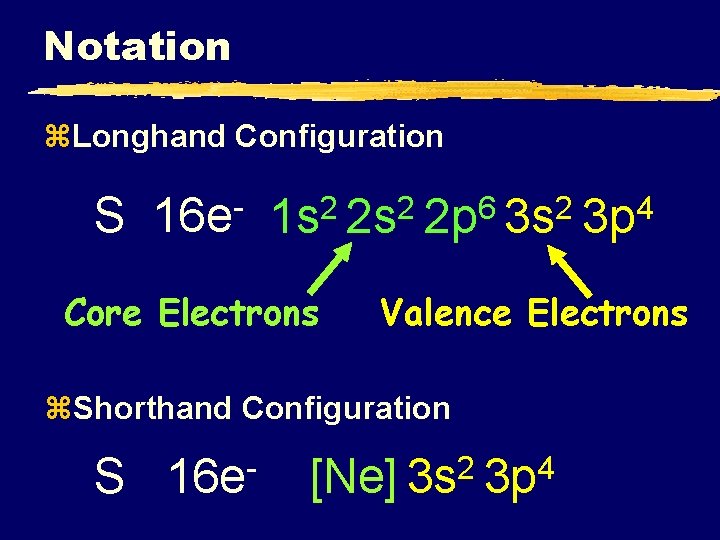
Notation z. Longhand Configuration S 16 e 6 2 2 2 1 s 2 s 2 p 3 s Core Electrons Valence Electrons z. Shorthand Configuration S 16 e 4 3 p 2 4 [Ne] 3 s 3 p

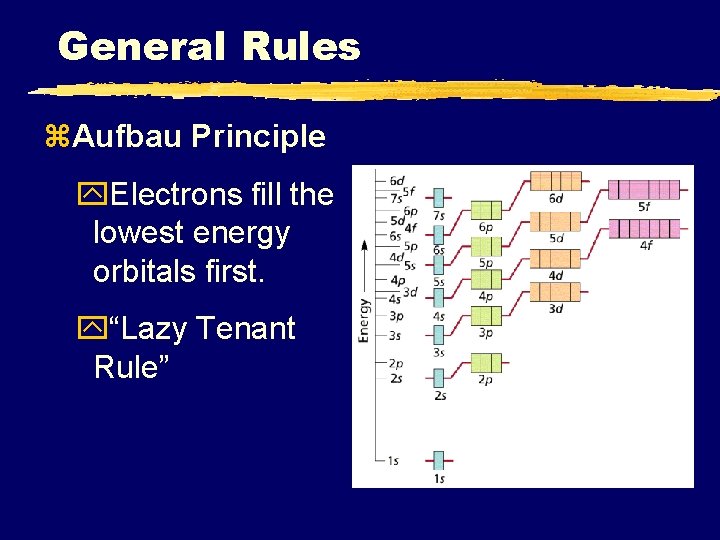
Filling Rules for Electron Orbitals Aufbau Principle: Electrons are added one at a time to the lowest energy orbitals available until all the electrons of the atom have been accounted for. Pauli Exclusion Principle: An orbital can hold a maximum of two electrons. To occupy the same orbital, two electrons must spin in opposite directions. Hund’s Rule: Electrons occupy equal-energy orbitals so that a maximum number of unpaired electrons results. *Aufbau is German for “building up”

General Rules z. Aufbau Principle y. Electrons fill the lowest energy orbitals first. y“Lazy Tenant Rule”

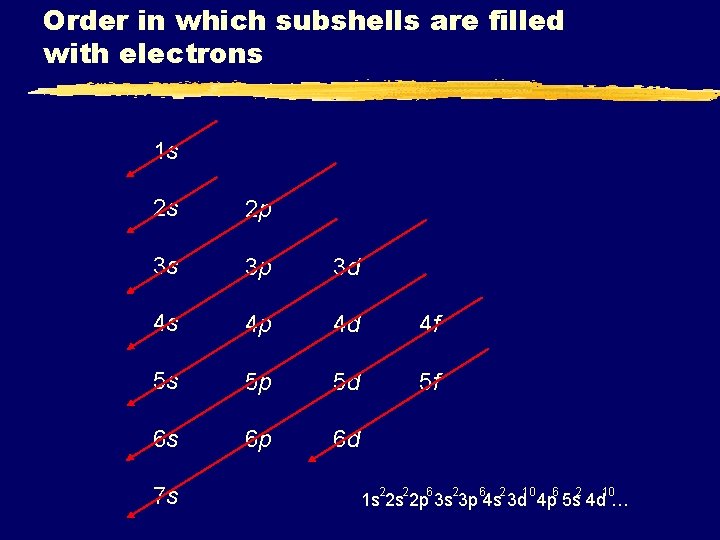
Order in which subshells are filled with electrons 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 5 s 5 p 5 d 5 f 6 s 6 p 6 d 7 s 2 2 6 2 10 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d …

Examples z. Chlorine z. Bromine z. Calcium

General Rules z. Pauli Exclusion Principle y. Each orbital can hold TWO electrons with opposite spins.

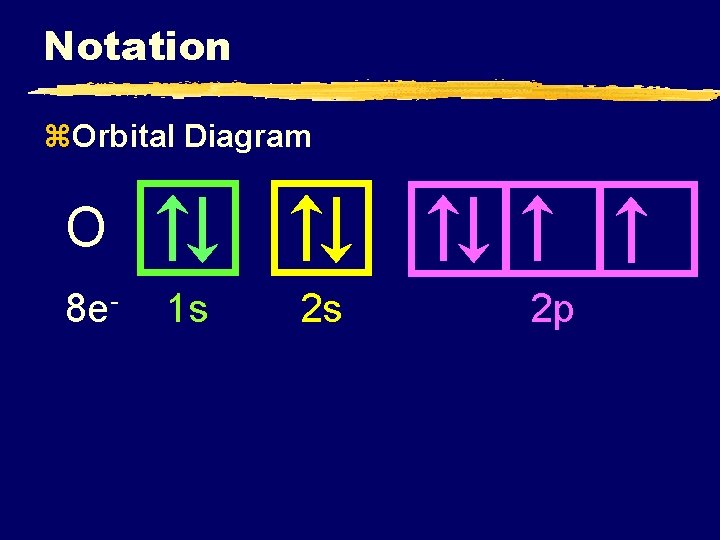
Notation z. Orbital Diagram O 8 e- 1 s 2 s 2 p

General Rules z. Hund’s Rule y. Within a sublevel, place one e- per orbital before pairing them. y“Empty Bus Seat Rule” WRONG RIGHT

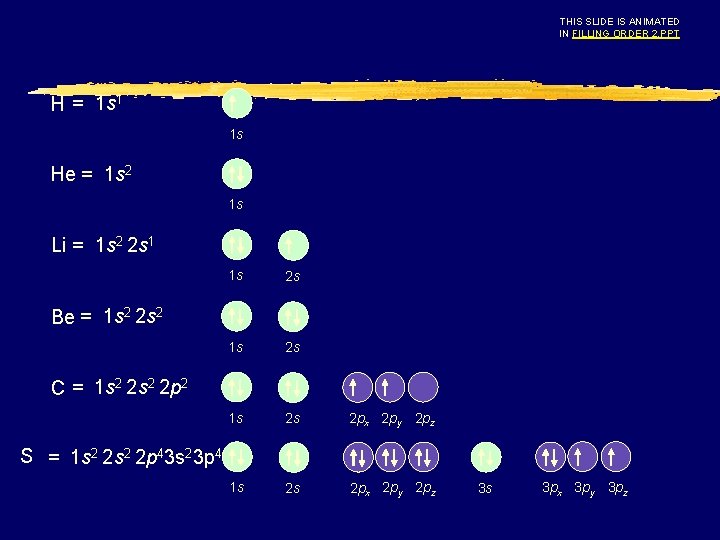
THIS SLIDE IS ANIMATED IN FILLING ORDER 2. PPT H = 1 s 1 1 s He = 1 s 2 1 s Li = 1 s 2 2 s 1 1 s 2 s 2 px 2 py 2 pz Be = 1 s 2 2 s 2 C = 1 s 2 2 p 2 S = 1 s 2 2 p 43 s 23 p 4 3 s 3 px 3 py 3 pz

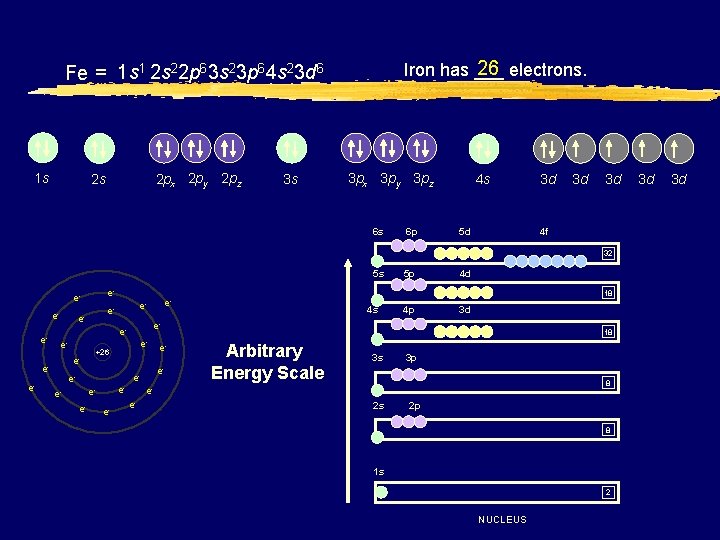
26 electrons. Iron has ___ Fe = 1 s 1 2 s 22 p 63 s 23 p 64 s 23 d 6 1 s 2 px 2 py 2 pz 2 s 3 s 3 px 3 py 3 pz 6 s 6 p 4 s 5 d 3 d 3 d 3 d 4 f 32 5 s e- eee- e- e- +26 e- e- ee- e- e- 4 s 4 p 3 d e- ee- e- e- 4 d 18 e- e- 5 p 18 Arbitrary Energy Scale 3 s 3 p 8 ee- 2 s 2 p 8 1 s 2 NUCLEUS 3 d 3 d

Examples z. Nitrogen z. Aluminum
![Shorthand Configuration Element symbol Electron configuration Ca Ar 4 s 2 V Ar 4 Shorthand Configuration Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] 4](https://slidetodoc.com/presentation_image/593ed8f33992ad27cb18762b25a714d7/image-17.jpg)
Shorthand Configuration Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] 4 s 2 3 d 3 F [He] 2 s 2 2 p 5 Ag [Kr] 5 s 2 4 d 9 I [Kr] 5 s 2 4 d 10 5 p 5 Xe [Kr] 5 s 2 4 d 10 5 p 6 Fe Sg 22 p 64 s [He] 2 s[Ar] 3 s 223 d 3 p 664 s 23 d 6 [Rn] 7 s 2 5 f 14 6 d 4