Electron Structure of the Atom Electron Configuration 7








- Slides: 73

Electron Structure of the Atom: Electron Configuration 7 -1

Rxn with O 2, CO 2 Density, MP, BP, polarity Such as Chemical Change Chemical Property Physical property Can be characterized by Can undergo changes such as Physical Change Such as Formation of H 2 O, H 2, O 2 Filtration, diffusion MATTER elements Solid Can be described by KMT Can be converted through melting into Liquid Can be in different states such as Can be classified into Can combine chemically into compounds Can be converted through evaporation into Gas mixtures Can combine physically into

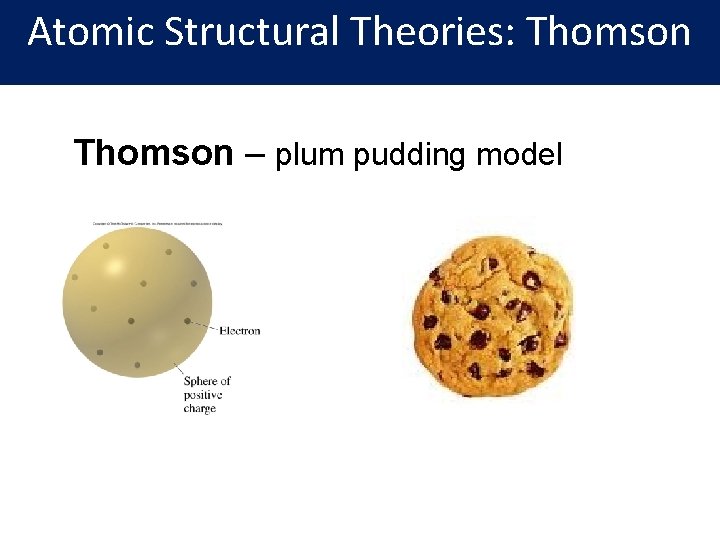
Atomic Structural Theories: Thomson – plum pudding model

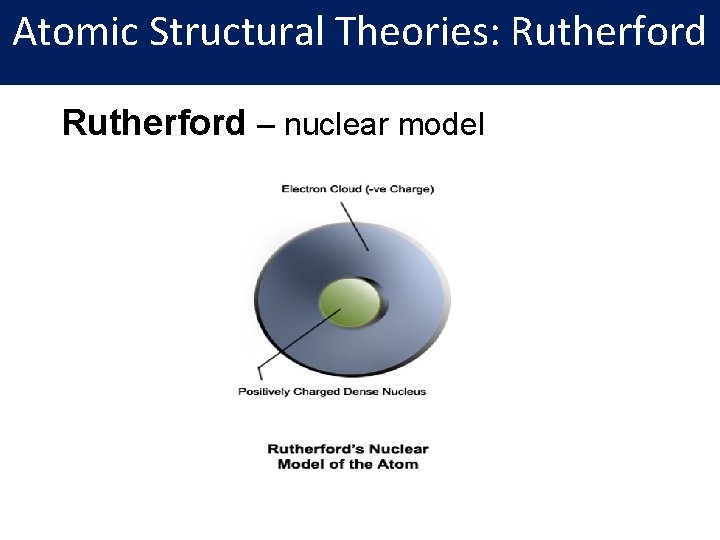
Atomic Structural Theories: Rutherford – nuclear model

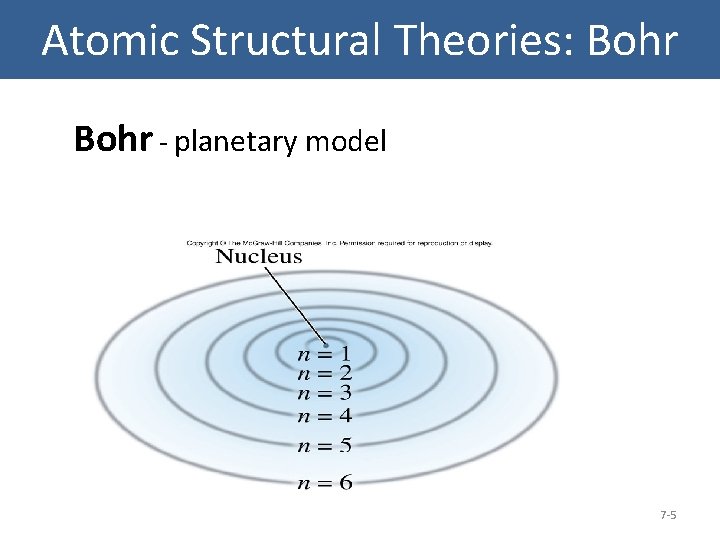
Atomic Structural Theories: Bohr el Bohr - planetary model 7 -5

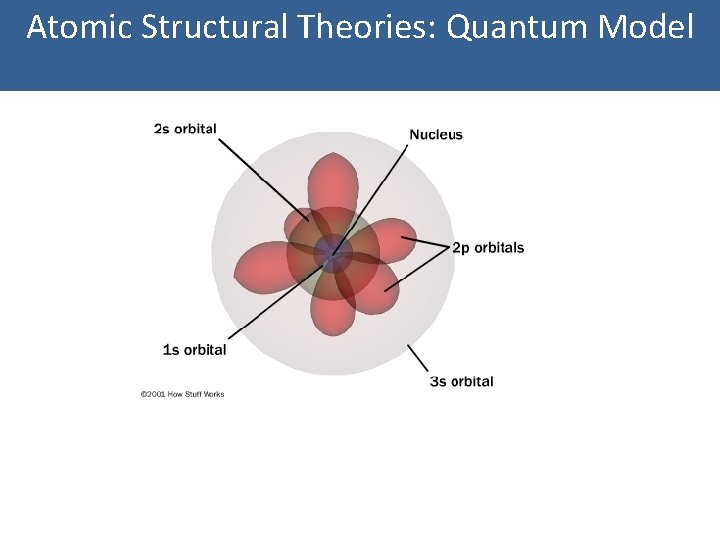
Atomic Structural Theories: Quantum Model

The Big Question How is the electron configuration of an atom written? 7 -7

Introductory Experiment Animation: fireworks 7 -8

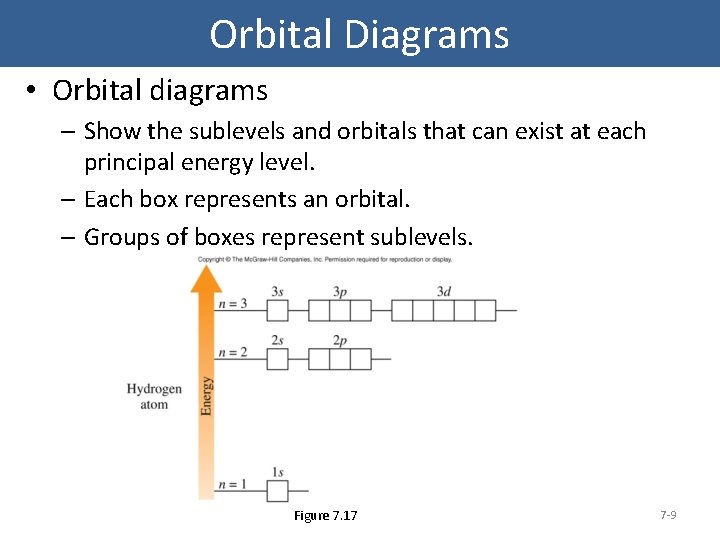
Orbital Diagrams • Orbital diagrams – Show the sublevels and orbitals that can exist at each principal energy level. – Each box represents an orbital. – Groups of boxes represent sublevels. Figure 7. 17 7 -9

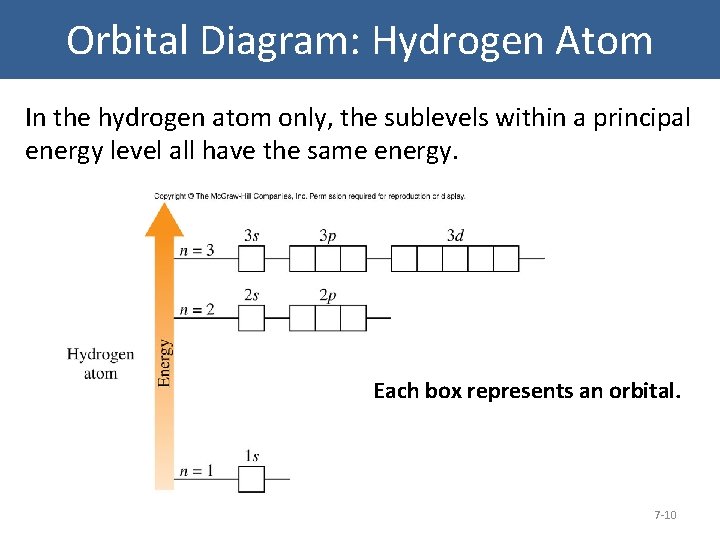
Orbital Diagram: Hydrogen Atom In the hydrogen atom only, the sublevels within a principal energy level all have the same energy. Each box represents an orbital. 7 -10

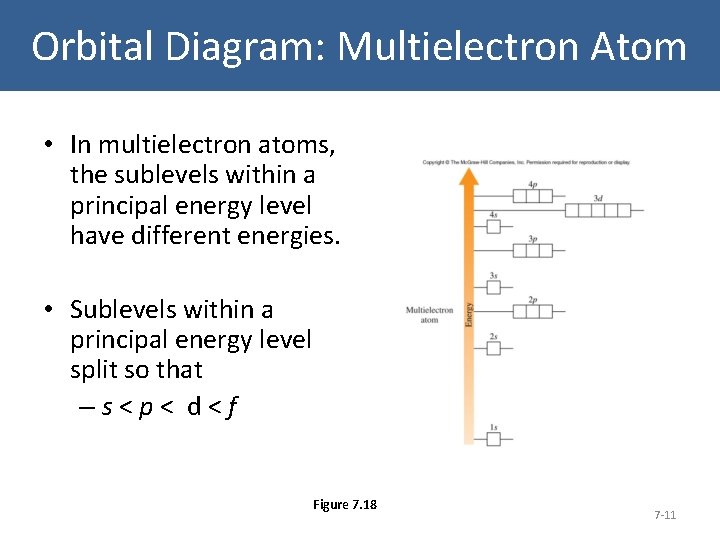
Orbital Diagram: Multielectron Atom • In multielectron atoms, the sublevels within a principal energy level have different energies. • Sublevels within a principal energy level split so that –s<p< d<f Figure 7. 18 7 -11

Orbital Diagram Rules • Two principles and one rule determine how the electrons are filled in the principal energy levels and sublevels. • Electrons are always filled in their ground state, or lowest energy state. 7 -12

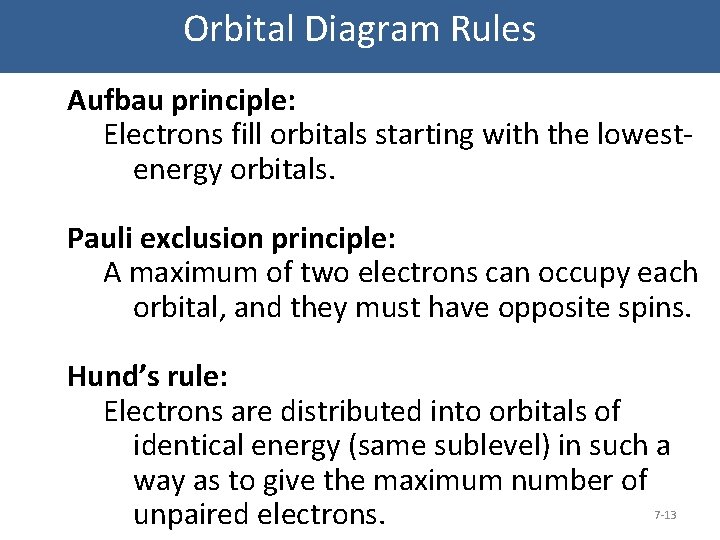
Orbital Diagram Rules Aufbau principle: Electrons fill orbitals starting with the lowestenergy orbitals. Pauli exclusion principle: A maximum of two electrons can occupy each orbital, and they must have opposite spins. Hund’s rule: Electrons are distributed into orbitals of identical energy (same sublevel) in such a way as to give the maximum number of unpaired electrons. 7 -13

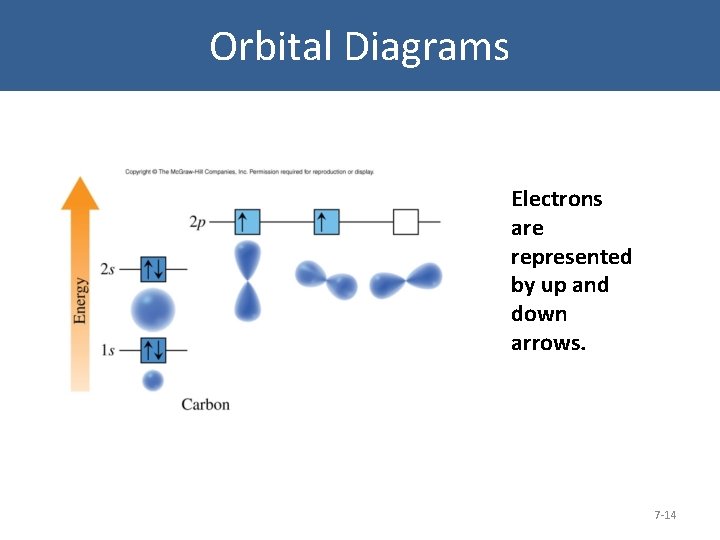
Orbital Diagrams Electrons are represented by up and down arrows. 7 -14

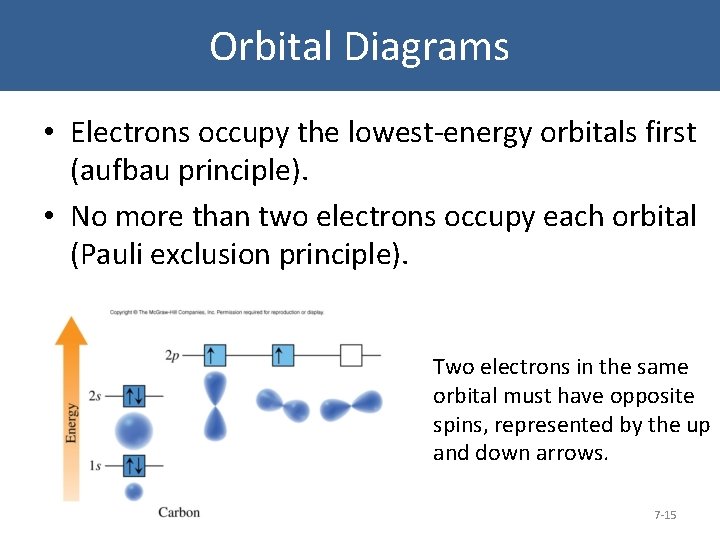
Orbital Diagrams • Electrons occupy the lowest-energy orbitals first (aufbau principle). • No more than two electrons occupy each orbital (Pauli exclusion principle). Two electrons in the same orbital must have opposite spins, represented by the up and down arrows. 7 -15

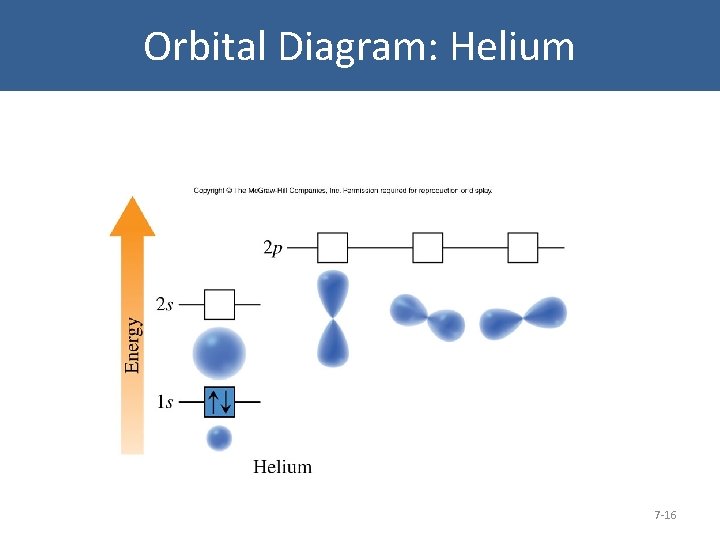
Orbital Diagram: Helium 7 -16

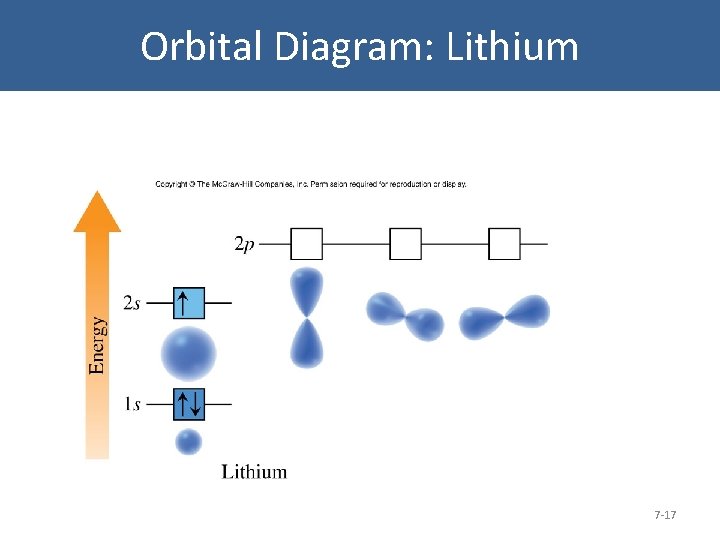
Orbital Diagram: Lithium 7 -17

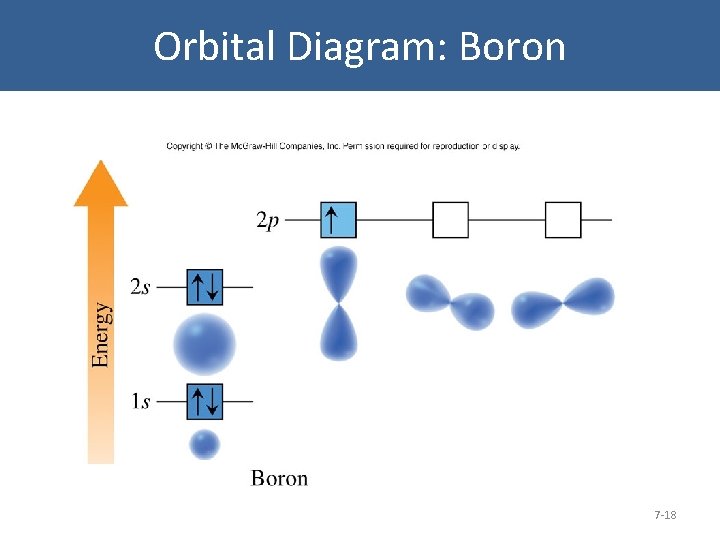
Orbital Diagram: Boron 7 -18

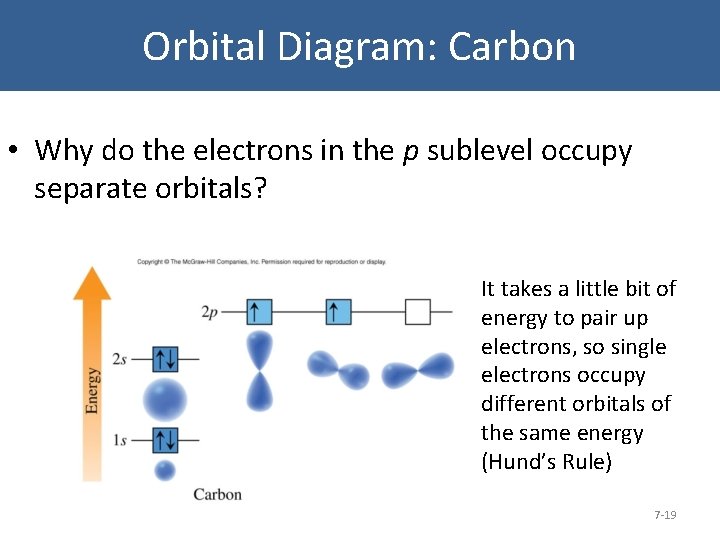
Orbital Diagram: Carbon • Why do the electrons in the p sublevel occupy separate orbitals? It takes a little bit of energy to pair up electrons, so single electrons occupy different orbitals of the same energy (Hund’s Rule) 7 -19

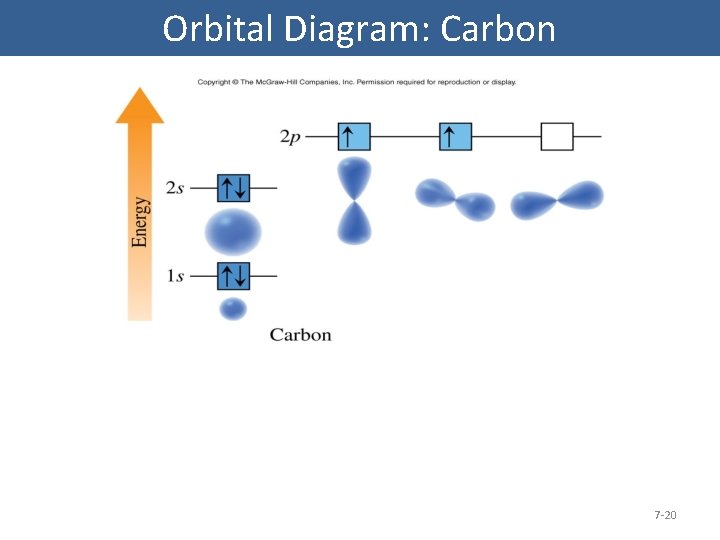
Orbital Diagram: Carbon 7 -20

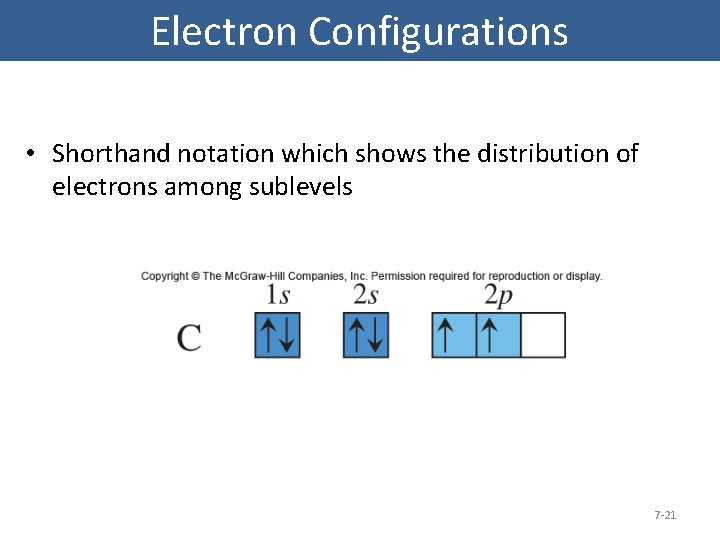
Electron Configurations • Shorthand notation which shows the distribution of electrons among sublevels 7 -21

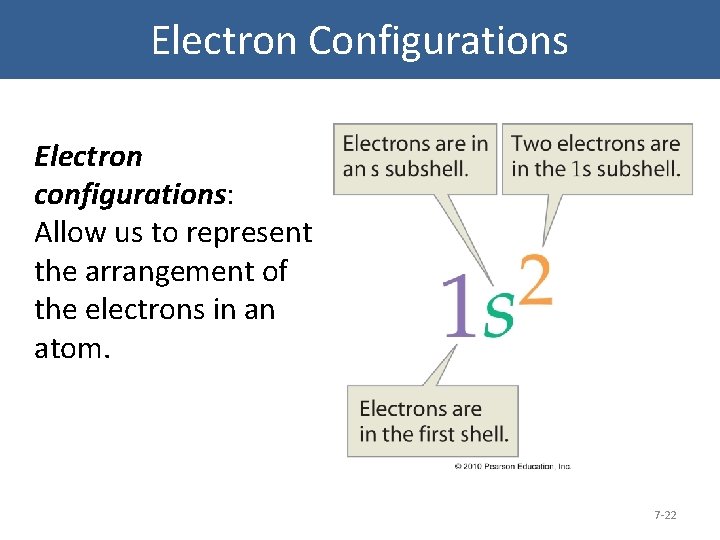
Electron Configurations Electron configurations: Allow us to represent the arrangement of the electrons in an atom. 7 -22

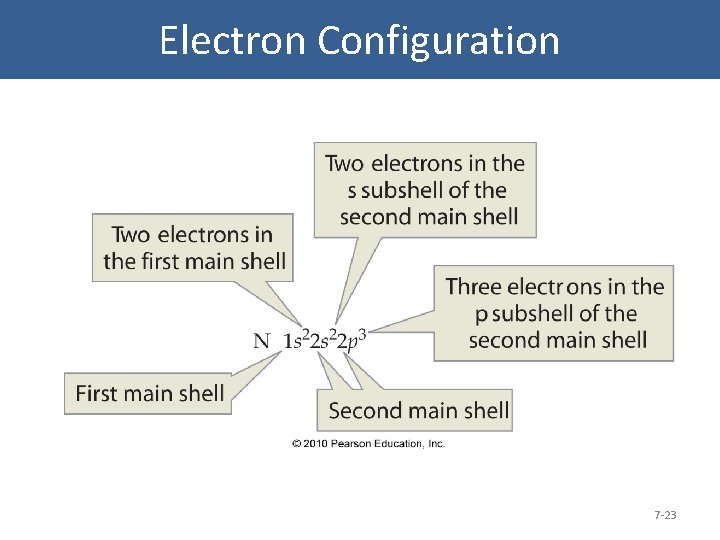
Electron Configuration 7 -23

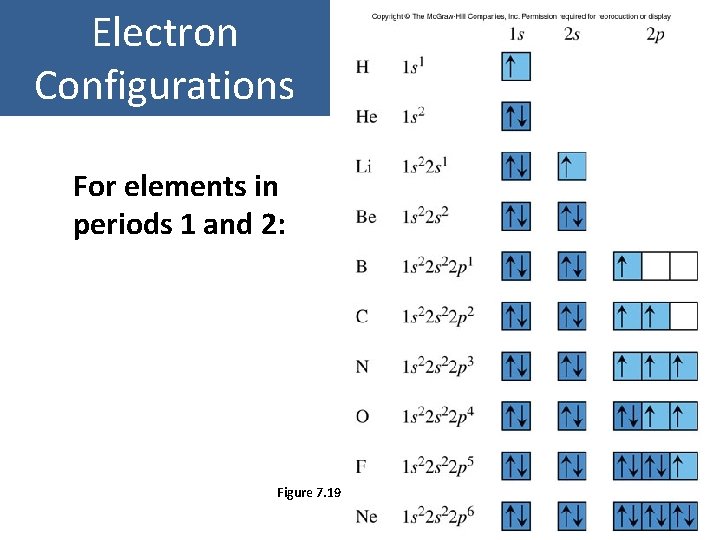
Electron Configurations For elements in periods 1 and 2: Figure 7. 19 7 -24

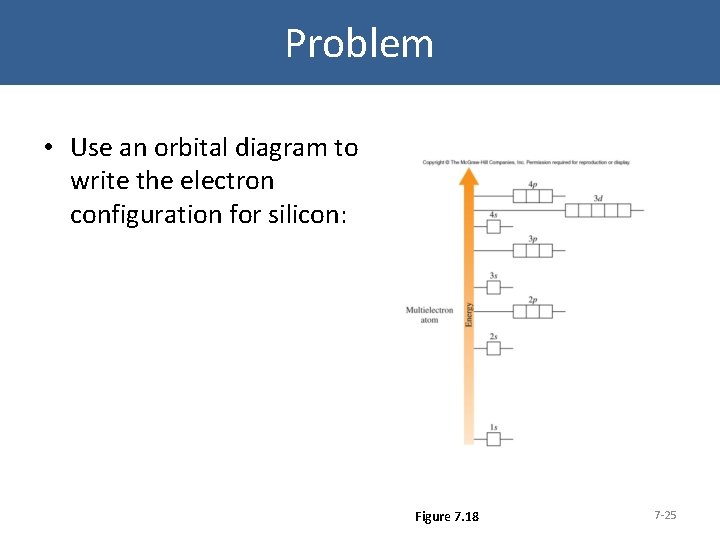
Problem • Use an orbital diagram to write the electron configuration for silicon: Figure 7. 18 7 -25

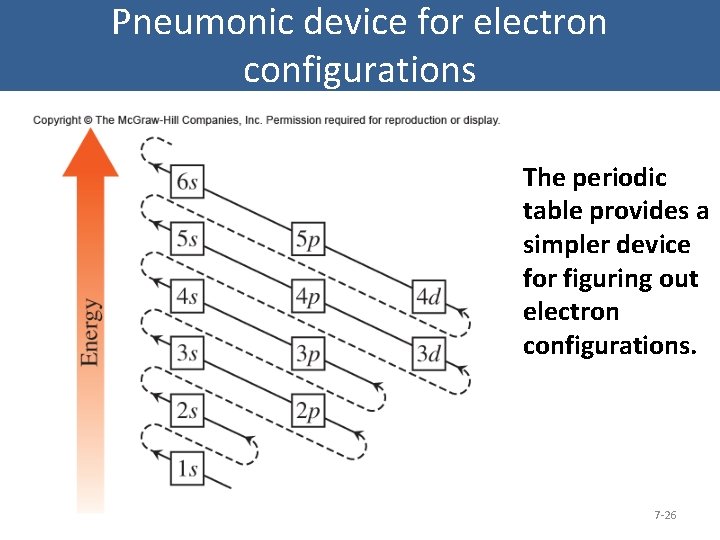
Pneumonic device for electron configurations The periodic table provides a simpler device for figuring out electron configurations. 7 -26

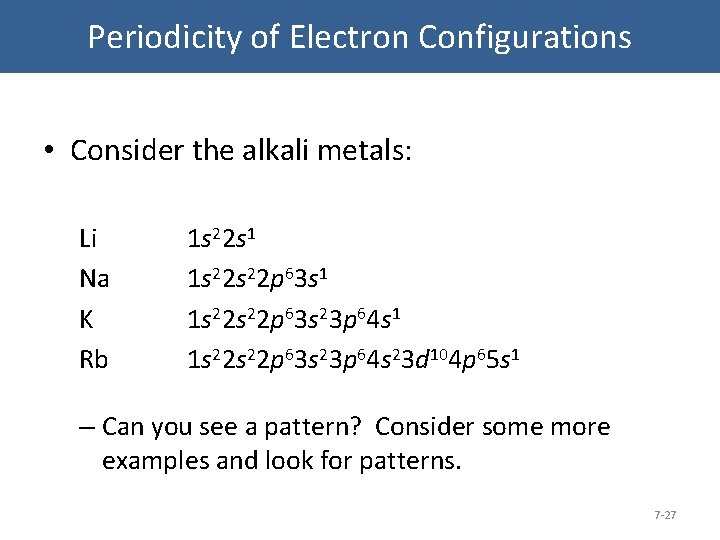
Periodicity of Electron Configurations • Consider the alkali metals: Li Na K Rb 1 s 22 s 1 1 s 22 s 22 p 63 s 23 p 64 s 1 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 1 – Can you see a pattern? Consider some more examples and look for patterns. 7 -27

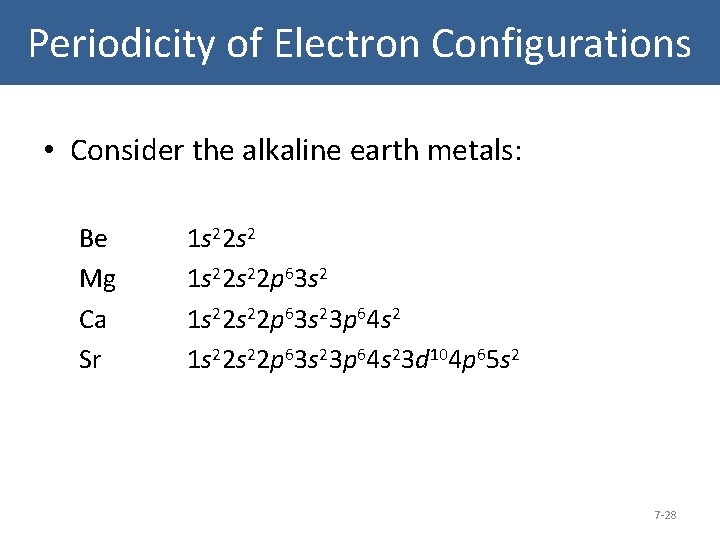
Periodicity of Electron Configurations • Consider the alkaline earth metals: Be Mg Ca Sr 1 s 22 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 2 7 -28

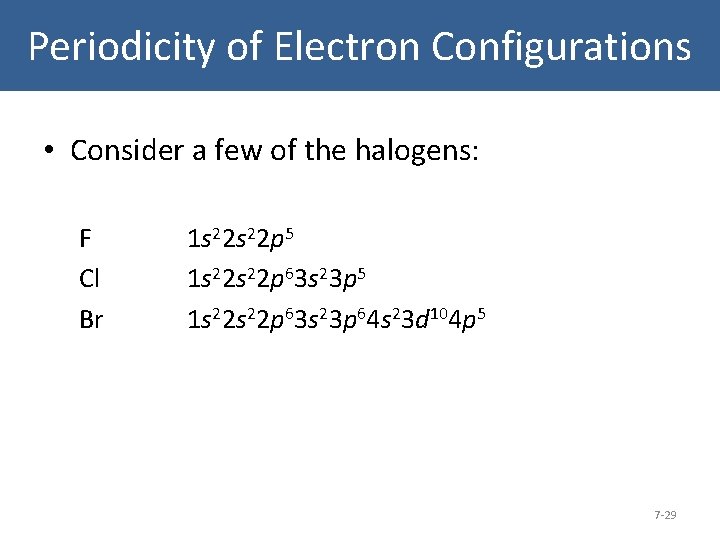
Periodicity of Electron Configurations • Consider a few of the halogens: F Cl Br 1 s 22 p 5 1 s 22 s 22 p 63 s 23 p 64 s 23 d 104 p 5 7 -29

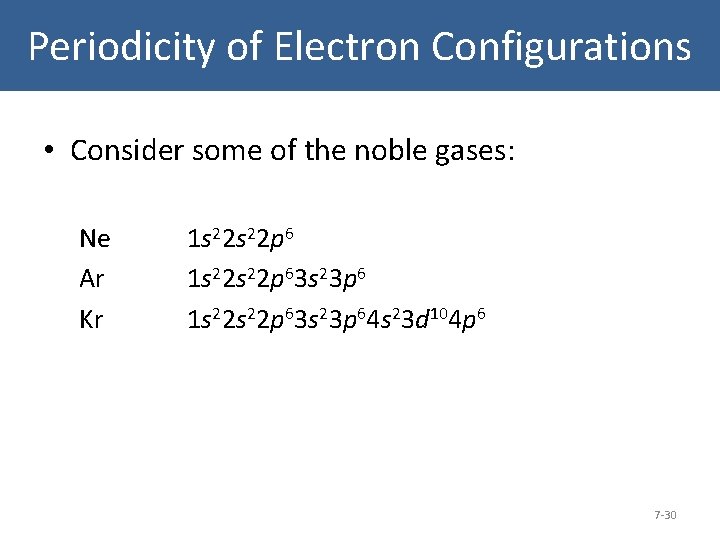
Periodicity of Electron Configurations • Consider some of the noble gases: Ne Ar Kr 1 s 22 s 22 p 63 s 23 p 6 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 7 -30

Conclusion Electron configuration and periodic table placement? 7 -31

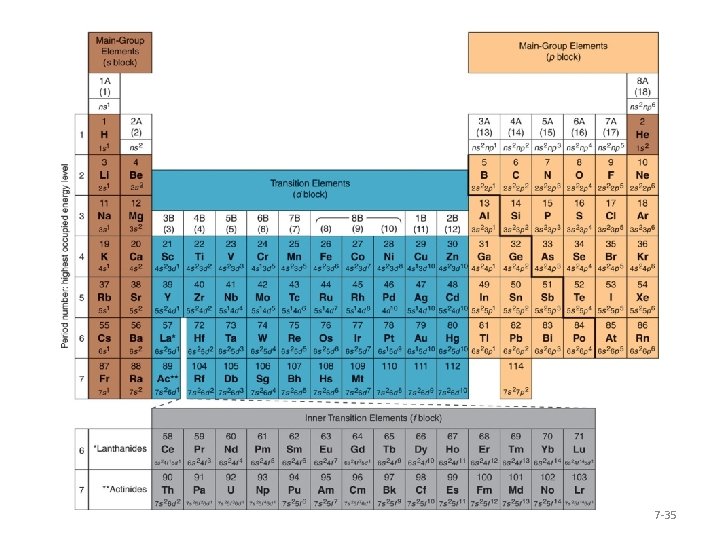
Periodicity of Electron Configurations • Notice that the number of columns in the s, p, d, and f blocks is the same as the number of electrons allowed in each sublevel. • This allows us to use the periodic table to write electron configurations without the aid of an orbital diagram. 7 -32

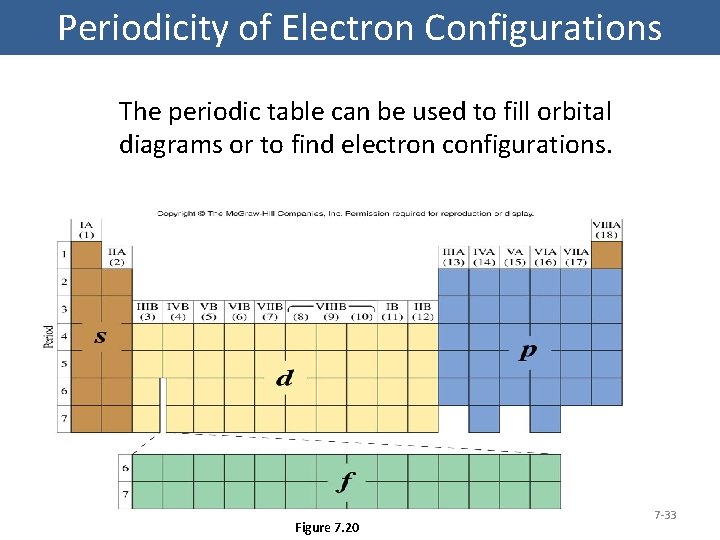
Periodicity of Electron Configurations The periodic table can be used to fill orbital diagrams or to find electron configurations. Figure 7. 20 7 -33

Periodicity of Electron Configurations • The principal energy level number, the number that comes before the sublevel letter designation, is the same as the period number for the s and p sublevels. • For the d sublevels, the principal energy level number is one less than the period number. Why? 7 -34

7 -35

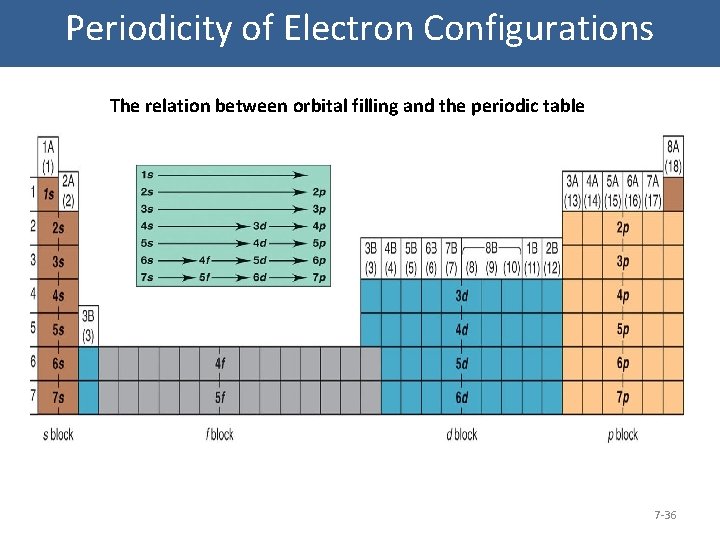
Periodicity of Electron Configurations The relation between orbital filling and the periodic table 7 -36

Periodicity of Electron Configurations Figure 7. 21 7 -37

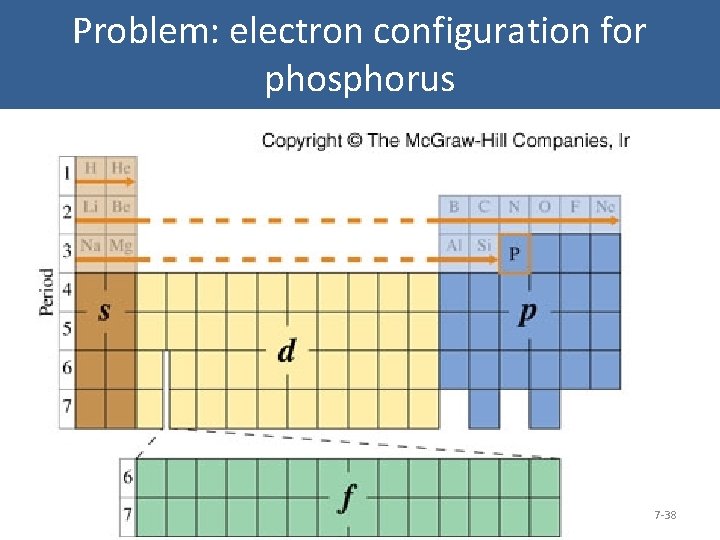
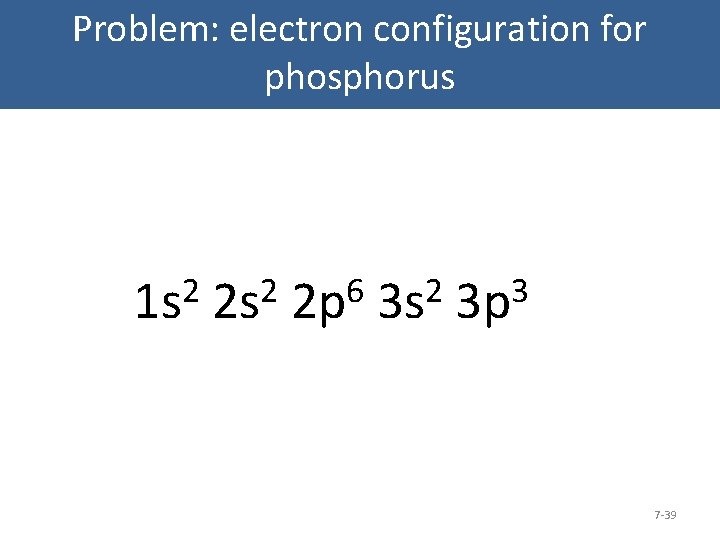
Problem: electron configuration for phosphorus 7 -38

Problem: electron configuration for phosphorus 2 1 s Orbital Diagram: Carbon 2 2 s 6 2 p 2 3 s 3 3 p 7 -39

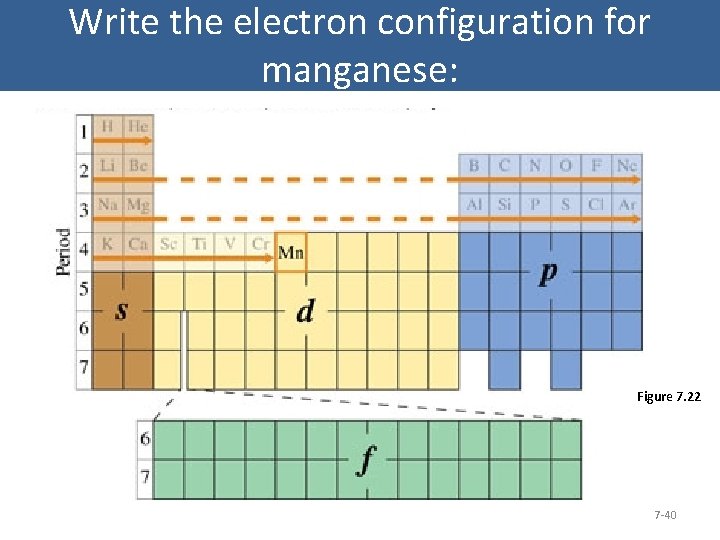
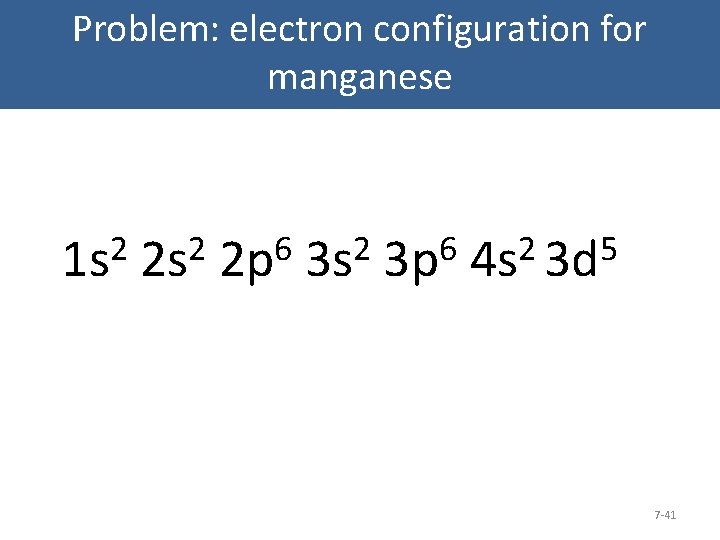
Write the electron configuration for manganese: Figure 7. 22 7 -40

Problem: electron configuration for manganese 2 1 s 2 2 s 6 2 6 Diagram: 3 p Carbon 2 p. Orbital 3 s 2 5 4 s 3 d 7 -41

Worksheet #2 -1 Electron Configuration I: Draw the electron configuration (long form) of the following elements just by looking at the periodic table. Na S Eu Ba F Ga Ar Sn Pb Mo U 7 -42

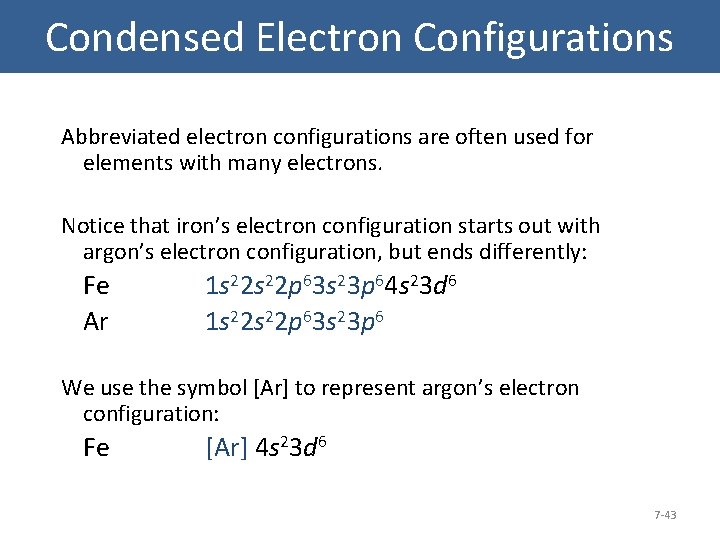
Condensed Electron Configurations Abbreviated electron configurations are often used for elements with many electrons. Notice that iron’s electron configuration starts out with argon’s electron configuration, but ends differently: Fe Ar 1 s 22 p 63 s 23 p 64 s 23 d 6 1 s 22 p 63 s 23 p 6 We use the symbol [Ar] to represent argon’s electron configuration: Fe [Ar] 4 s 23 d 6 7 -43

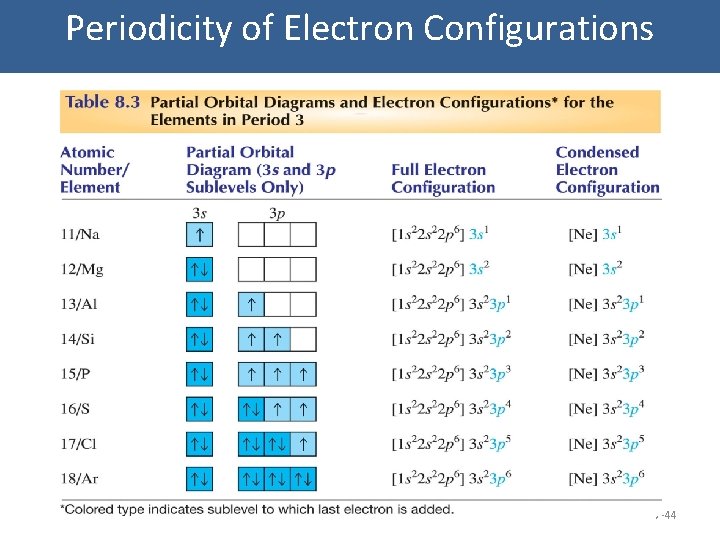
Periodicity of Electron Configurations 7 -44

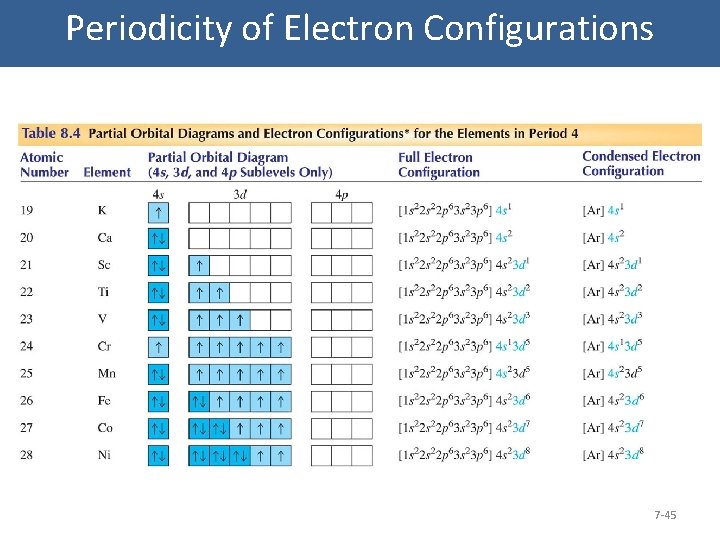
Periodicity of Electron Configurations 7 -45

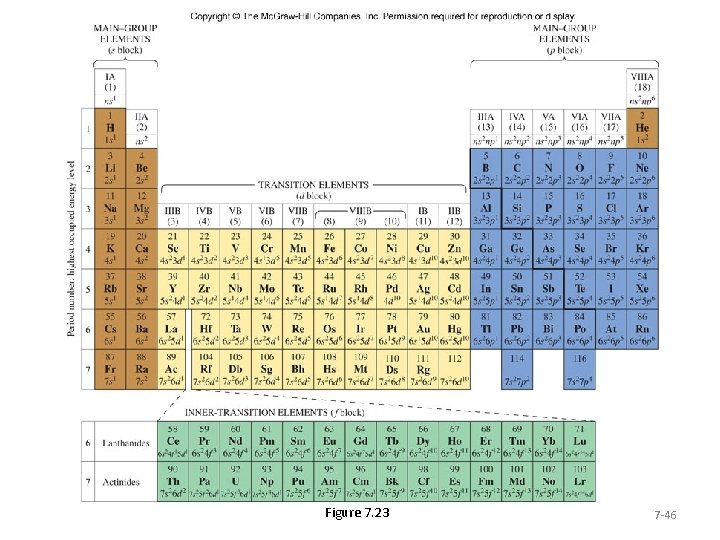
Figure 7. 23 7 -46

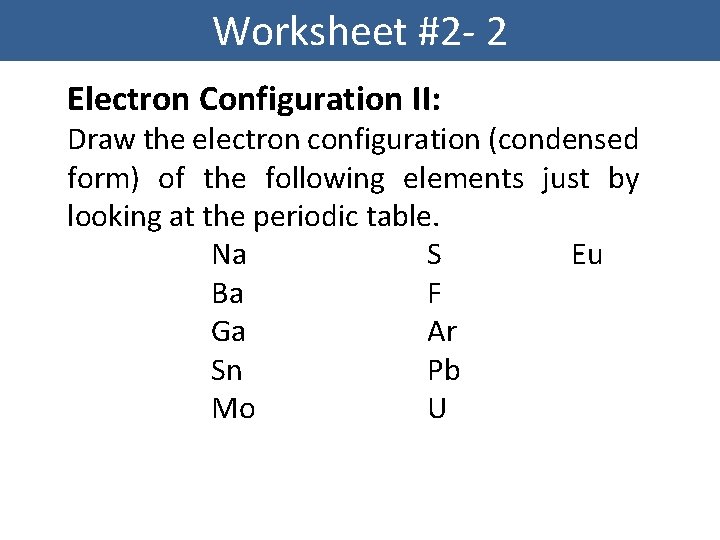
Worksheet #2 - 2 Electron Configuration II: Draw the electron configuration (condensed form) of the following elements just by looking at the periodic table. Na S Eu Ba F Ga Ar Sn Pb Mo U

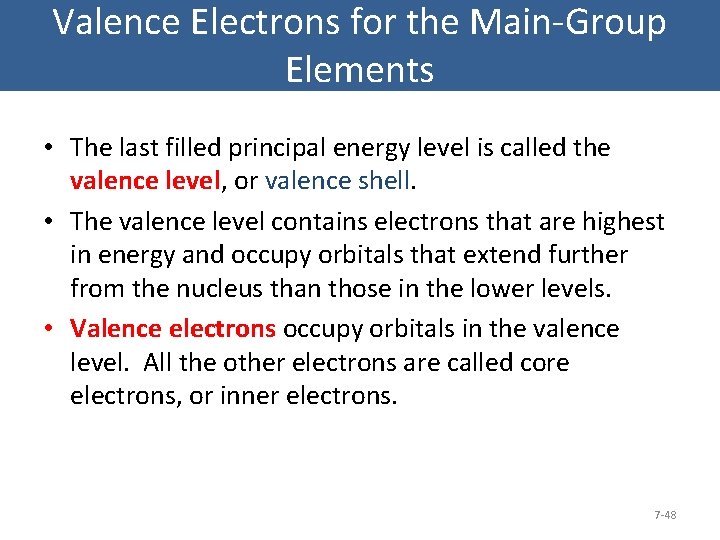
Valence Electrons for the Main-Group Elements • The last filled principal energy level is called the valence level, or valence shell. • The valence level contains electrons that are highest in energy and occupy orbitals that extend further from the nucleus than those in the lower levels. • Valence electrons occupy orbitals in the valence level. All the other electrons are called core electrons, or inner electrons. 7 -48

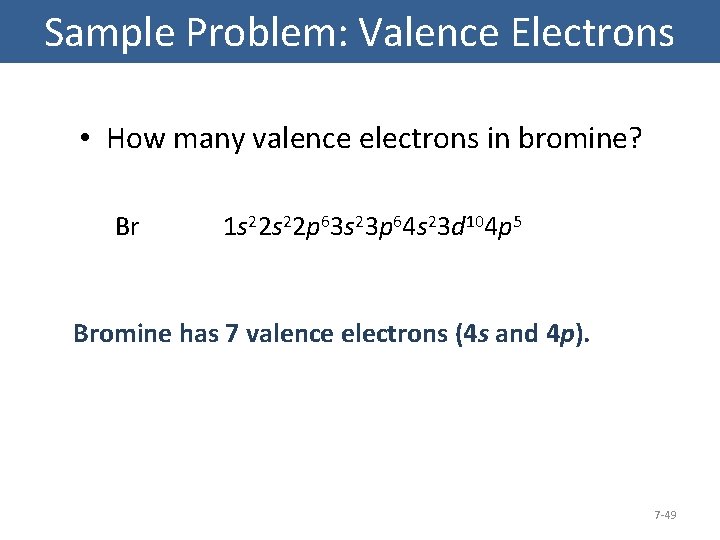
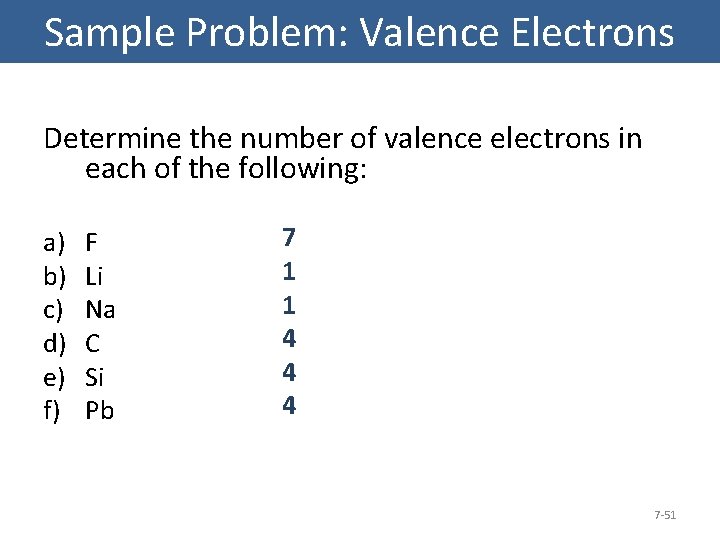
Sample Problem: Valence Electrons • How many valence electrons in bromine? Br 1 s 22 p 63 s 23 p 64 s 23 d 104 p 5 Bromine has 7 valence electrons (4 s and 4 p). 7 -49

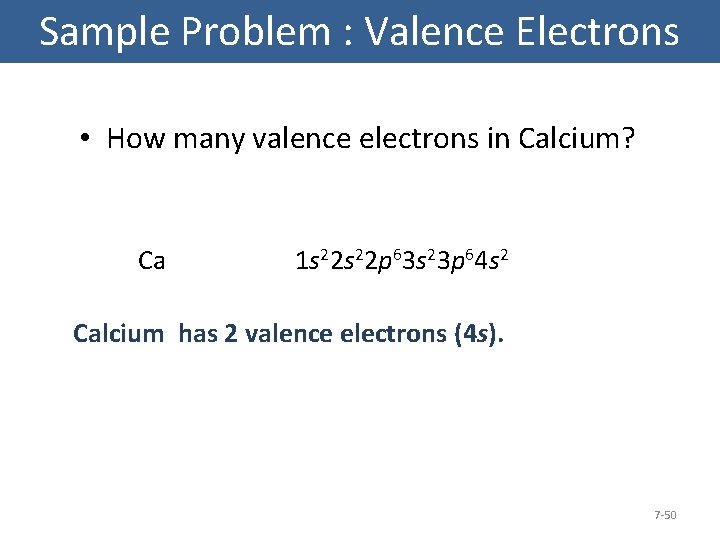
Sample Problem : Valence Electrons • How many valence electrons in Calcium? Ca 1 s 22 p 63 s 23 p 64 s 2 Calcium has 2 valence electrons (4 s). 7 -50

Sample Problem: Valence Electrons Determine the number of valence electrons in each of the following: a) b) c) d) e) f) F Li Na C Si Pb 7 1 1 4 4 4 7 -51

Conclusion Electronic configuration? Valence electron? Family Number? 7 -52

Conclusion The valence electrons of family A elements are the same as the family number. They can be seen in the outer most energy levels in the electron configuration of the element. The same is not true for family B elements.

Electron Configurations for Ions • In atoms, the number of electrons is equal to the number of protons, which is the atomic number. • In ions, the number of electrons does not equal the atomic number. We must add or subtract electrons, depending on whether the ion is an anion or cation. 7 -54

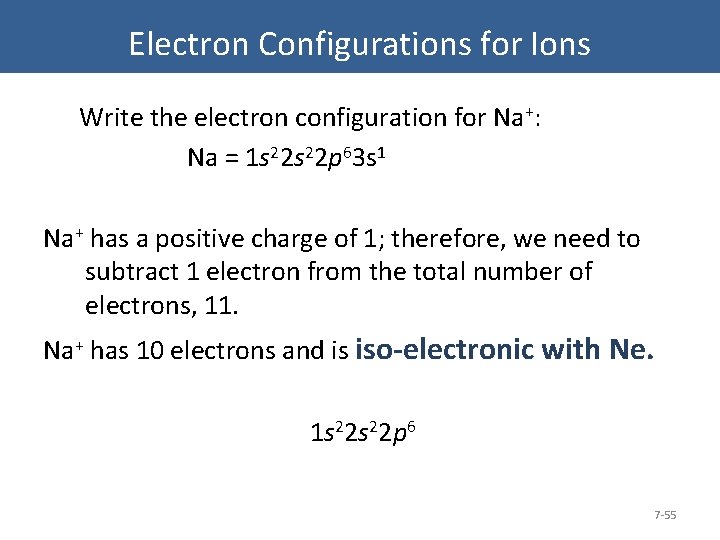
Electron Configurations for Ions Write the electron configuration for Na+: Na = 1 s 22 p 63 s 1 Na+ has a positive charge of 1; therefore, we need to subtract 1 electron from the total number of electrons, 11. Na+ has 10 electrons and is iso-electronic with Ne. 1 s 22 p 6 7 -55

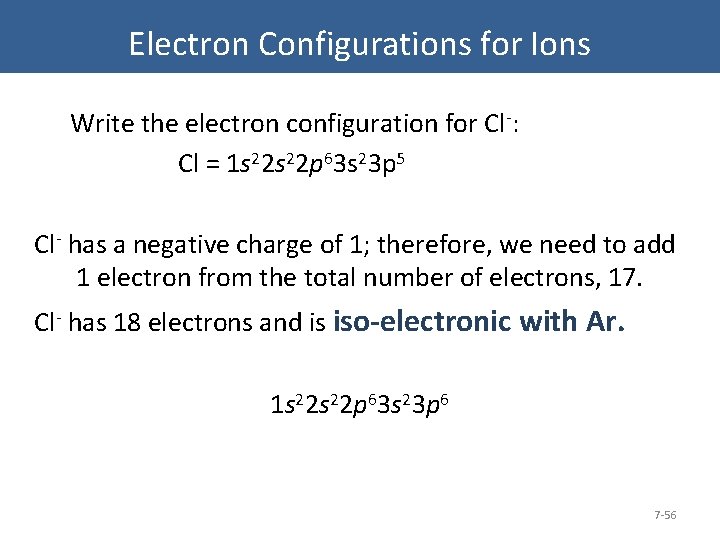
Electron Configurations for Ions Write the electron configuration for Cl-: Cl = 1 s 22 p 63 s 23 p 5 Cl- has a negative charge of 1; therefore, we need to add 1 electron from the total number of electrons, 17. Cl- has 18 electrons and is iso-electronic with Ar. 1 s 22 p 63 s 23 p 6 7 -56

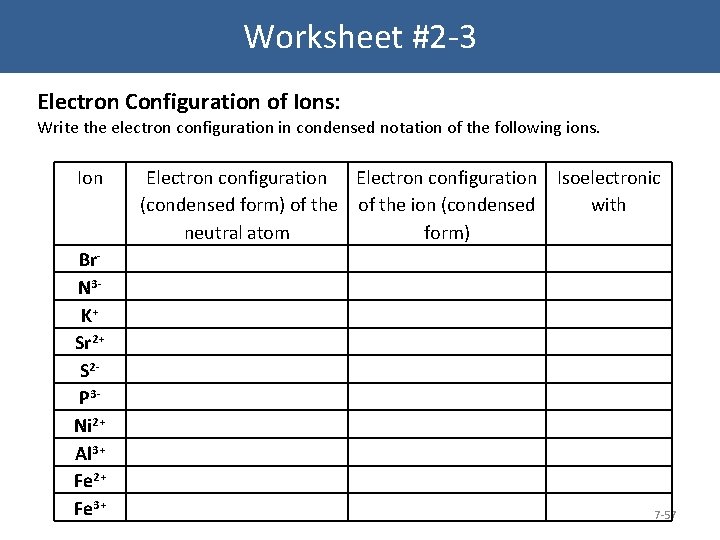
Worksheet #2 -3 Electron Configuration of Ions: Write the electron configuration in condensed notation of the following ions. Ion Br. N 3 - K+ Sr 2+ S 2 - P 3 Ni 2+ Al 3+ Fe 2+ Fe 3+ Electron configuration Isoelectronic (condensed form) of the ion (condensed with neutral atom form) 7 -57

Conclusion Electron configuration of ions? Family number? Isoelectronic with? 7 -58

Conclusion Family A atoms form ions so as to have the same electron configuration as a noble gas element. The charges of the ions formed can be determined from the family number of the element.

Conclusion For family B metals, the electrons lost in ion formation come from the highest energy level filled (not the d-electrons yet)

Quantum Numbers Each electron has its own set of four quantum numbers. These quantum numbers describe the location and the shape of the electron’s orbital.

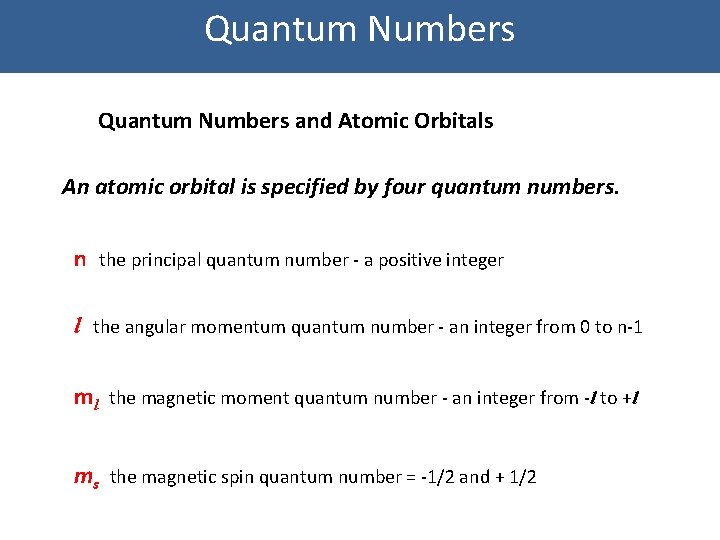
Quantum Numbers and Atomic Orbitals An atomic orbital is specified by four quantum numbers. n the principal quantum number - a positive integer l the angular momentum quantum number - an integer from 0 to n-1 ml the magnetic moment quantum number - an integer from -l to +l ms the magnetic spin quantum number = -1/2 and + 1/2

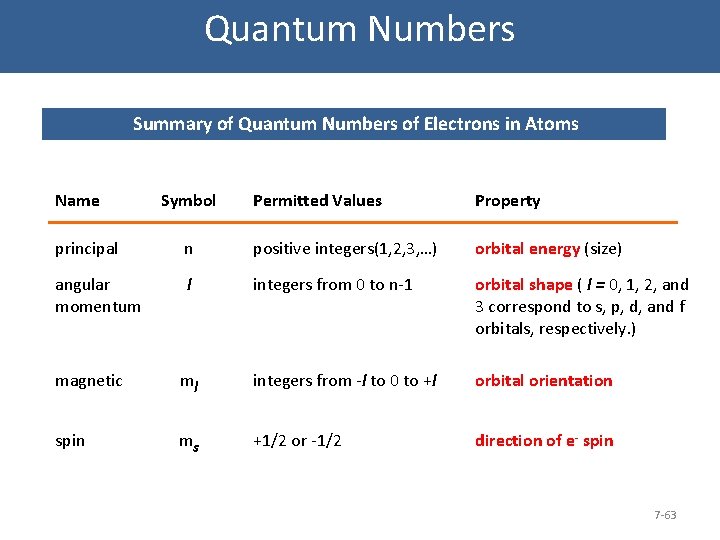
Quantum Numbers Summary of Quantum Numbers of Electrons in Atoms Name Symbol Permitted Values Property principal n positive integers(1, 2, 3, …) orbital energy (size) angular momentum l integers from 0 to n-1 orbital shape ( l = 0, 1, 2, and 3 correspond to s, p, d, and f orbitals, respectively. ) magnetic ml integers from -l to 0 to +l orbital orientation spin ms +1/2 or -1/2 direction of e- spin 7 -63

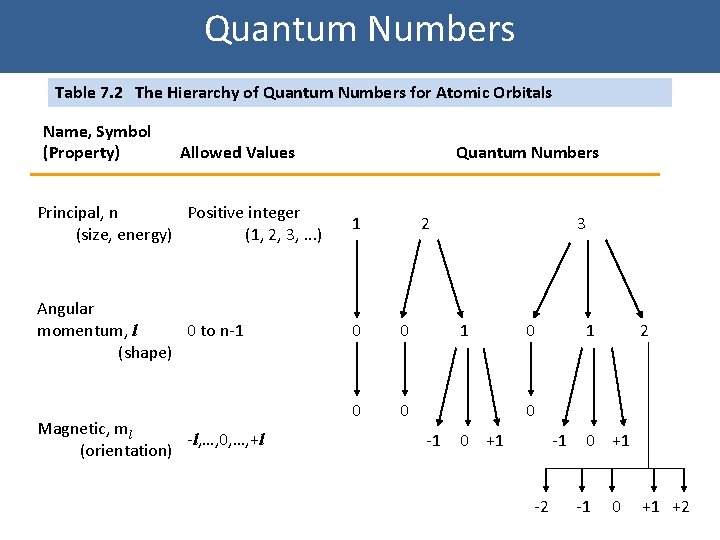
Quantum Numbers Table 7. 2 The Hierarchy of Quantum Numbers for Atomic Orbitals Name, Symbol (Property) Allowed Values Quantum Numbers Principal, n Positive integer (size, energy) (1, 2, 3, . . . ) 1 Angular momentum, l 0 to n-1 (shape) 0 0 Magnetic, ml -l, …, 0, …, +l (orientation) 2 3 1 0 1 2 0 -1 0 +1 -1 -2 0 +1 -1 0 +1 +2

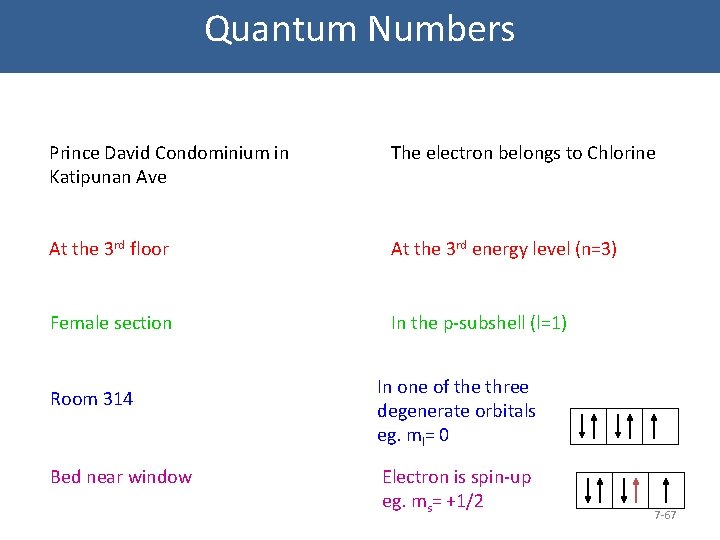
Quantum Numbers • Where can you find Donna? • Donna lives in a dorm in a condominium unit. In the dorm floor, there are male and female sections per floor. Per room, there are two beds only: one near the window, one not. If she is asked, where EXACTLY she lives/sleeps, how would she answer? Prince David Condominium in Katipunan Ave At the 3 rd floor Female section Room 314 Bed near window 7 -65

Quantum Numbers • Where can you find the electron? • The quantum mechanical model uses a certain way to describe an orbital. You can say you are asking the question, Where does my electron live/reside? We use three quantum numbers to describe an orbital. We add a fourth to pinpoint the electron in that orbital 1. 2. 3. 4. Principal quantum no. (n) Azimuthal or Angular Momentum quantum no. (l) Magnetic quantum no. (ml) Spin quantum no. (ms) 7 -66

Quantum Numbers Prince David Condominium in Katipunan Ave The electron belongs to Chlorine At the 3 rd floor At the 3 rd energy level (n=3) Female section In the p-subshell (l=1) Room 314 Bed near window In one of the three degenerate orbitals eg. ml= 0 Electron is spin-up eg. ms= +1/2 7 -67

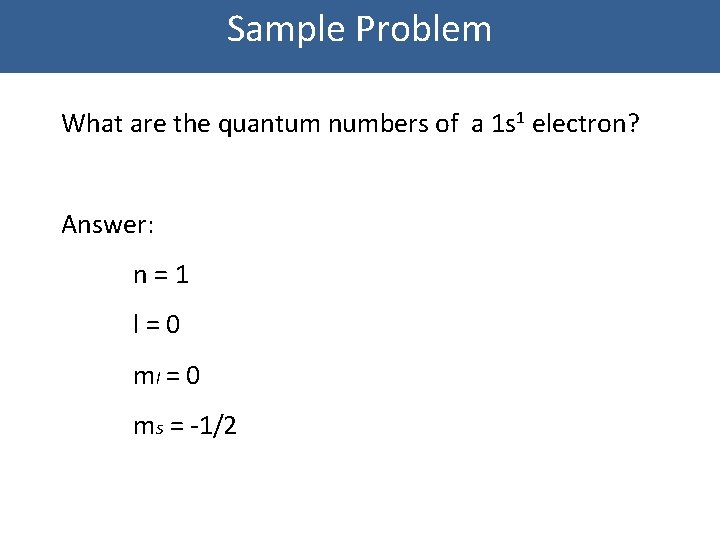
Sample Problem What are the quantum numbers of a 1 s 1 electron? Answer: n=1 l=0 ml = 0 ms = -1/2

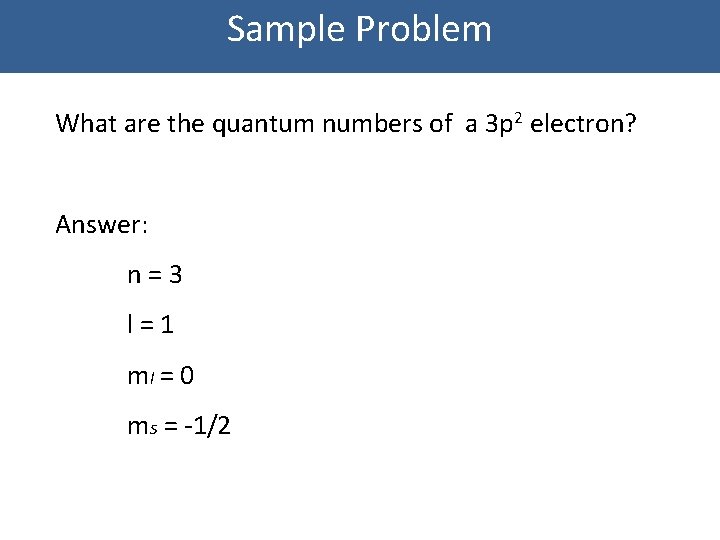
Sample Problem What are the quantum numbers of a 3 p 2 electron? Answer: n=3 l=1 ml = 0 ms = -1/2

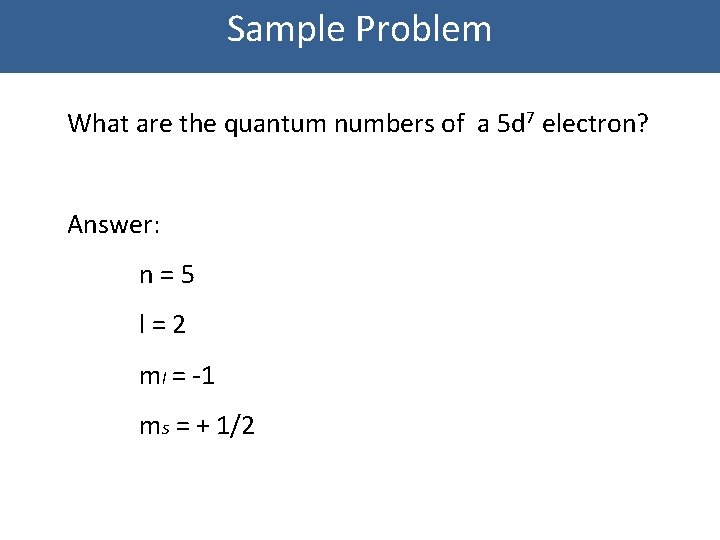
Sample Problem What are the quantum numbers of a 5 d 7 electron? Answer: n=5 l=2 ml = -1 ms = + 1/2

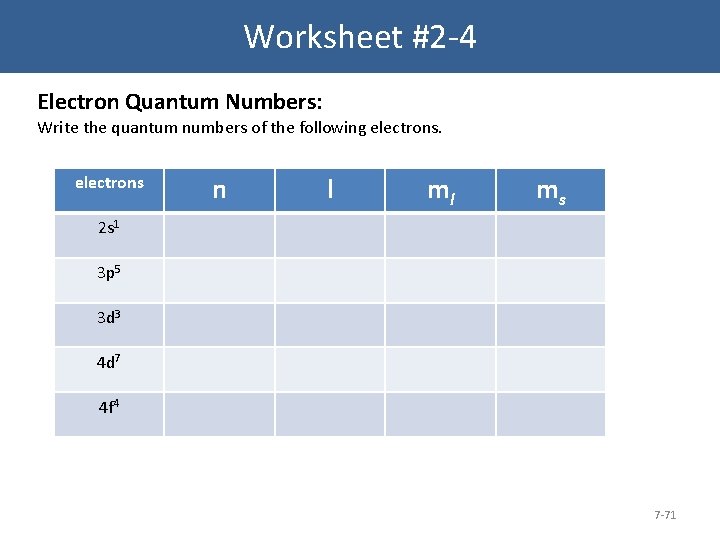
Worksheet #2 -4 Electron Quantum Numbers: Write the quantum numbers of the following electrons n l ml ms 2 s 1 3 p 5 3 d 3 4 d 7 4 f 4 7 -71

Summary of Electron Configuration Concepts 1. Long form 2. Condensed form 3. Ions, iso-electronic, family A and B elements 4. Family Number/Period Number 5. Valence electrons 6. Quantum numbers

Points to Ponder…. . • Is there a proton configuration or a neutron configuration? Why or why not? • How do scientists synthesize new elements? • What are the newest four elements added to the periodic table? • Is there a limit to the number of elements that scientists can synthesize?