Valence Electrons What is a Valence Electron Valence





- Slides: 16

Valence Electrons


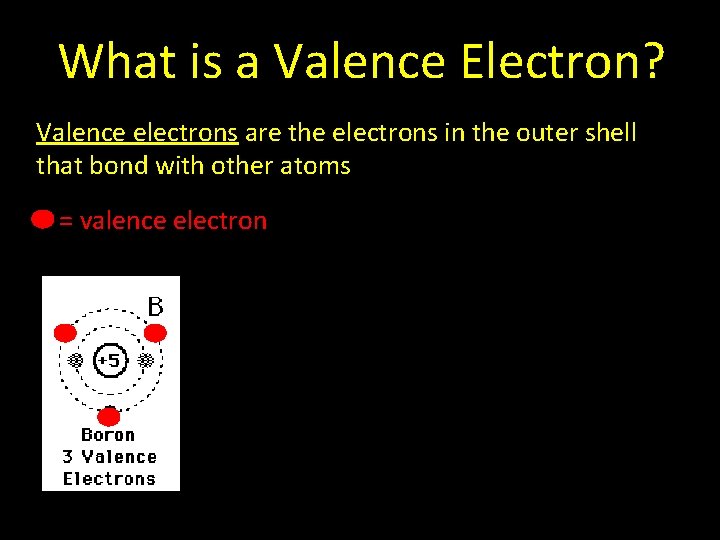
What is a Valence Electron? Valence electrons are the electrons in the outer shell that bond with other atoms

What is a Valence Electron? Valence electrons are the electrons in the outer shell that bond with other atoms = valence electron

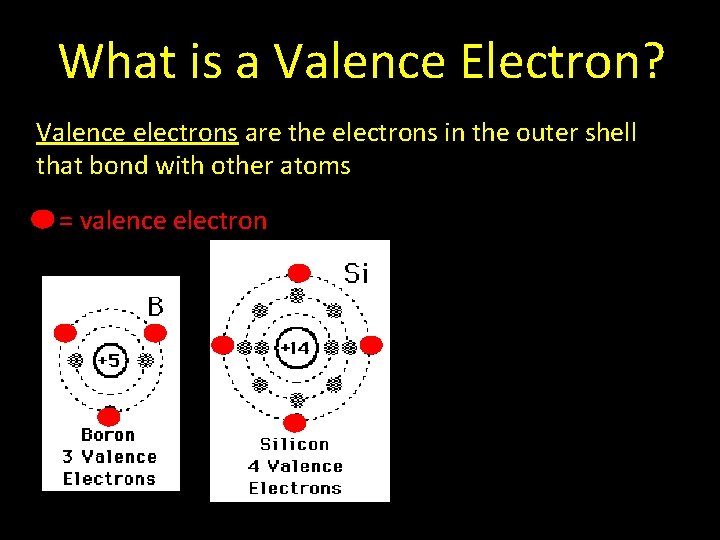
What is a Valence Electron? Valence electrons are the electrons in the outer shell that bond with other atoms = valence electron

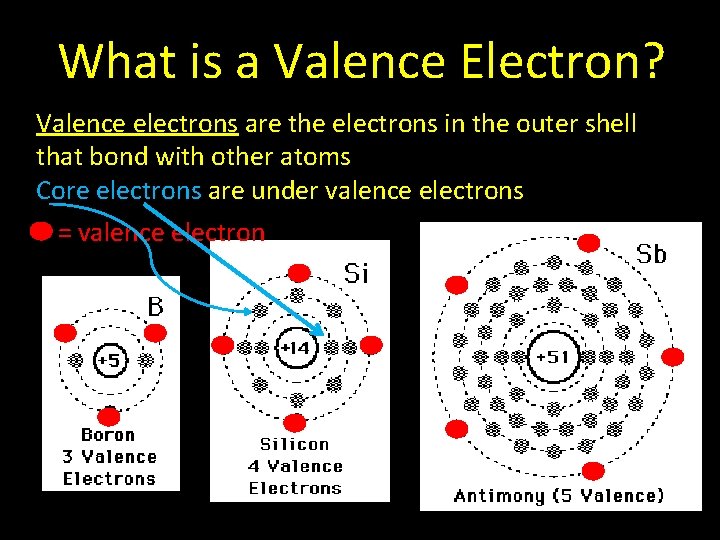
What is a Valence Electron? Valence electrons are the electrons in the outer shell that bond with other atoms Core electrons are under valence electrons = valence electron

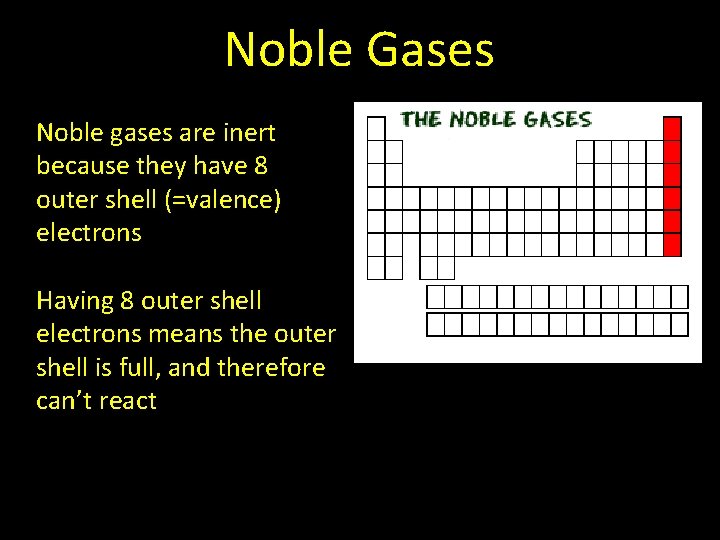
Outer Shells of Electrons The number of valence electrons determines an element’s chemical properties For example, the noble gases on the right side of the periodic table are inert Inert = do not react with other atoms

Noble Gases Noble gases are inert because they have 8 outer shell (=valence) electrons Having 8 outer shell electrons means the outer shell is full, and therefore can’t react

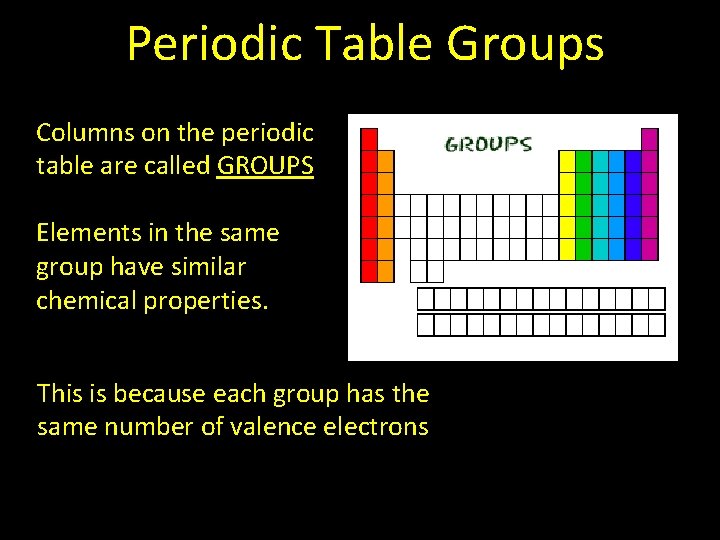
Periodic Table Groups Columns on the periodic table are called GROUPS Elements in the same group have similar chemical properties. This is because each group has the same number of valence electrons

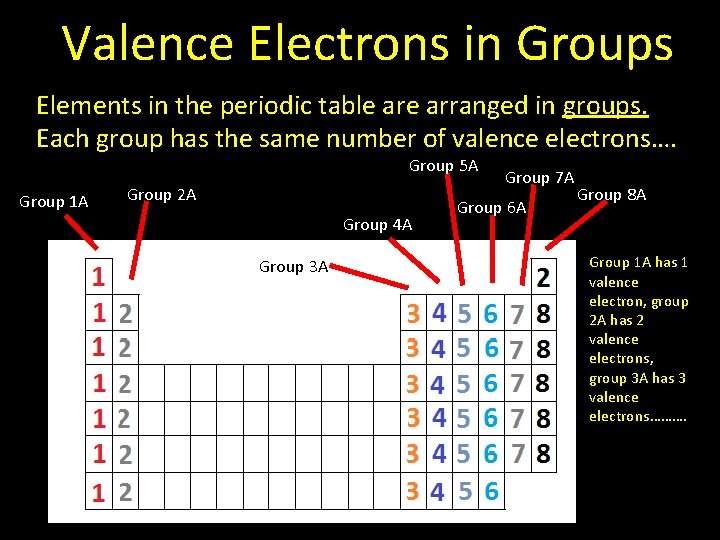
Valence Electrons in Groups Elements in the periodic table arranged in groups. Each group has the same number of valence electrons…. Group 5 A Group 1 A Group 2 A Group 4 A Group 3 A Group 7 A Group 6 A Group 8 A Group 1 A has 1 valence electron, group 2 A has 2 valence electrons, group 3 A has 3 valence electrons……….

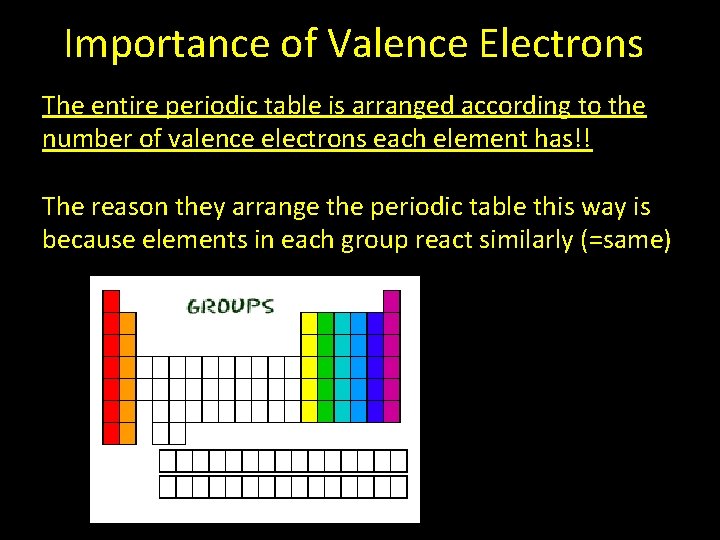
Importance of Valence Electrons The entire periodic table is arranged according to the number of valence electrons each element has!! The reason they arrange the periodic table this way is because elements in each group react similarly (=same)

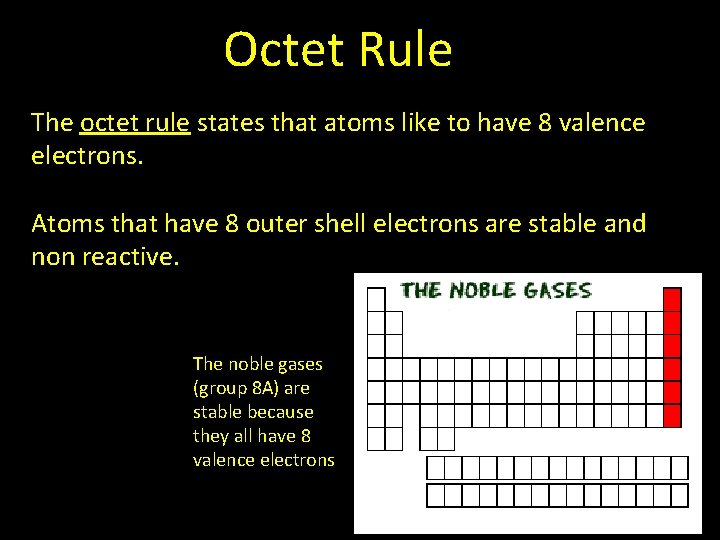
Octet Rule The octet rule states that atoms like to have 8 valence electrons. Atoms that have 8 outer shell electrons are stable and non reactive. The noble gases (group 8 A) are stable because they all have 8 valence electrons

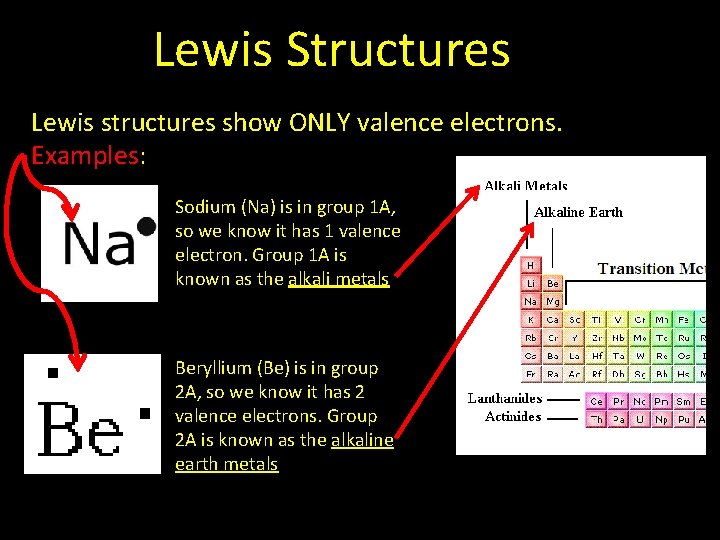
Lewis Structures Lewis structures show ONLY valence electrons. Examples: Sodium (Na) is in group 1 A, so we know it has 1 valence electron. Group 1 A is known as the alkali metals Beryllium (Be) is in group 2 A, so we know it has 2 valence electrons. Group 2 A is known as the alkaline earth metals

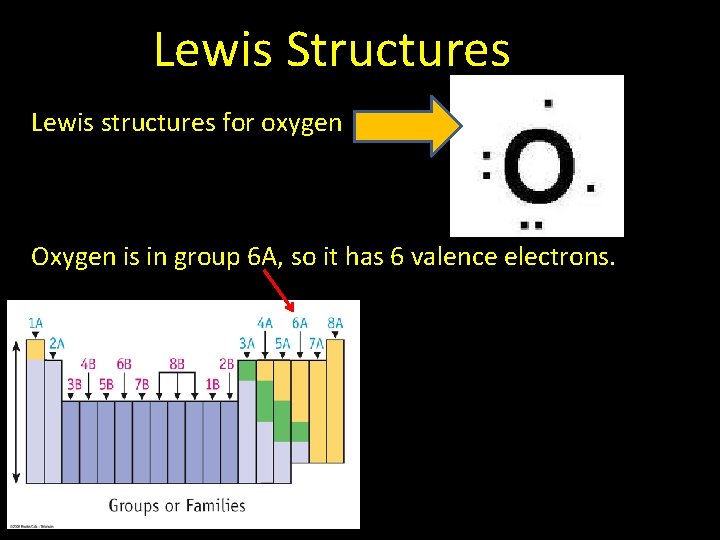
Lewis Structures Lewis structures for oxygen Oxygen is in group 6 A, so it has 6 valence electrons.

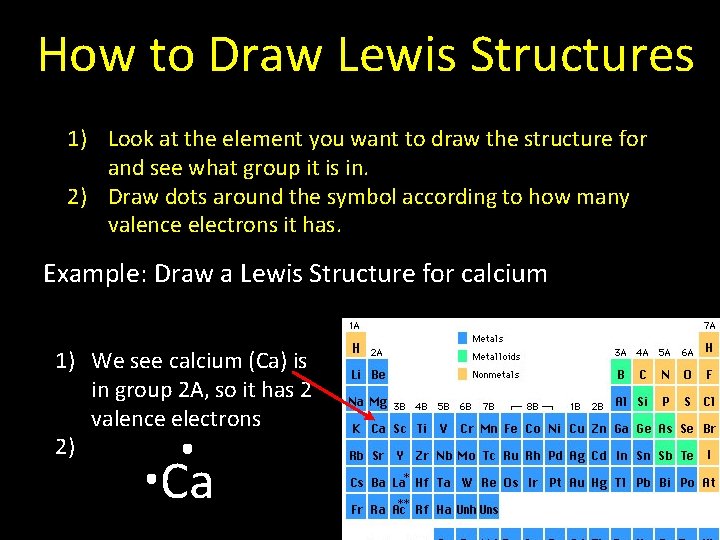
How to Draw Lewis Structures 1) Look at the element you want to draw the structure for and see what group it is in. 2) Draw dots around the symbol according to how many valence electrons it has. Example: Draw a Lewis Structure for calcium 1) We see calcium (Ca) is in group 2 A, so it has 2 valence electrons 2) Ca

Questions 1. What is a valence electron? Draw an atom and show the valence electrons. 2. How many valence electrons do the following elements have: Si, C, O, Ar, K 3. What does inert mean? 4. How many valence electrons do atoms like to have? 5. What do “groups” on the periodic table have in common? 6. Draw the Lewis structure for the following elements: Ne, P, Mg, Na, Pb, Sb