ELECTRONS IN THE ATOM ELECTRON CONFIGURATIONS THE ADDRESSES

![ELECTRON APARTMENTS The apartment building has different floors [principal energy level], different apartments on ELECTRON APARTMENTS The apartment building has different floors [principal energy level], different apartments on](https://slidetodoc.com/presentation_image/14344375854d0d1c0e6504d647fb3ddc/image-10.jpg)

- Slides: 27

ELECTRONS IN THE ATOM ELECTRON CONFIGURATIONS “THE ADDRESSES OF ELEMENTS”

EQ’s • What do the chemical properties of atoms depend on? • What is the quantum mechanical model? • How is the quantum mechanical model organized? • What is the Aufbau Principle?

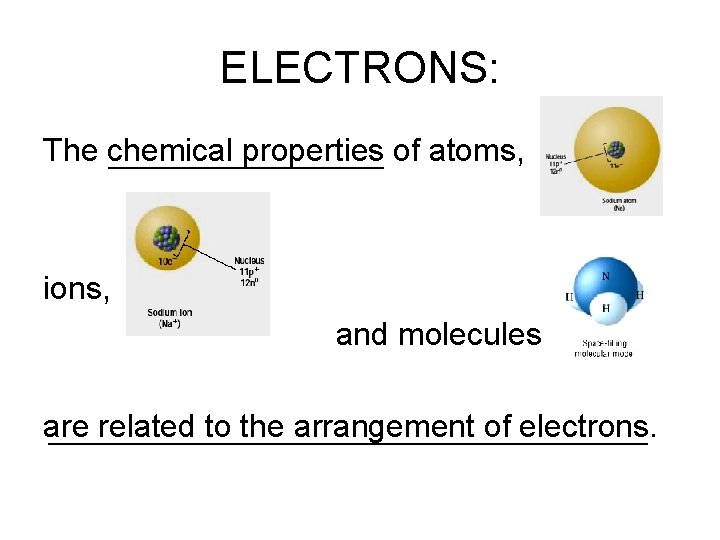
ELECTRONS: The chemical properties of atoms, ions, and molecules are related to the arrangement of electrons.

EVOLUTION OF THE ATOM To understand this concept, let’s take a look at the history of the atomic models. Dalton – solid indivisible mass

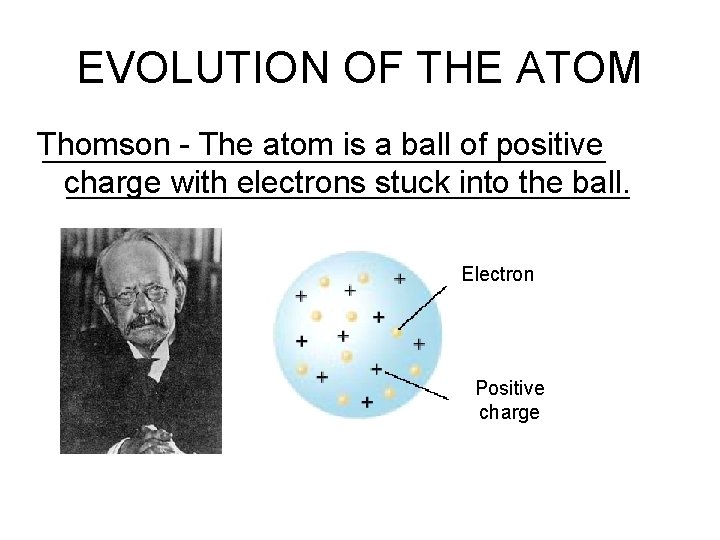
EVOLUTION OF THE ATOM Thomson - The atom is a ball of positive charge with electrons stuck into the ball. Electron Positive charge

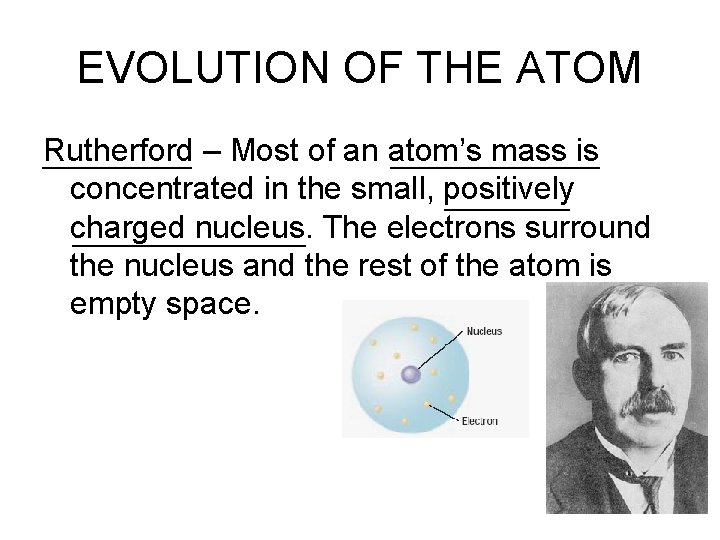
EVOLUTION OF THE ATOM Rutherford – Most of an atom’s mass is concentrated in the small, positively charged nucleus. The electrons surround the nucleus and the rest of the atom is empty space.

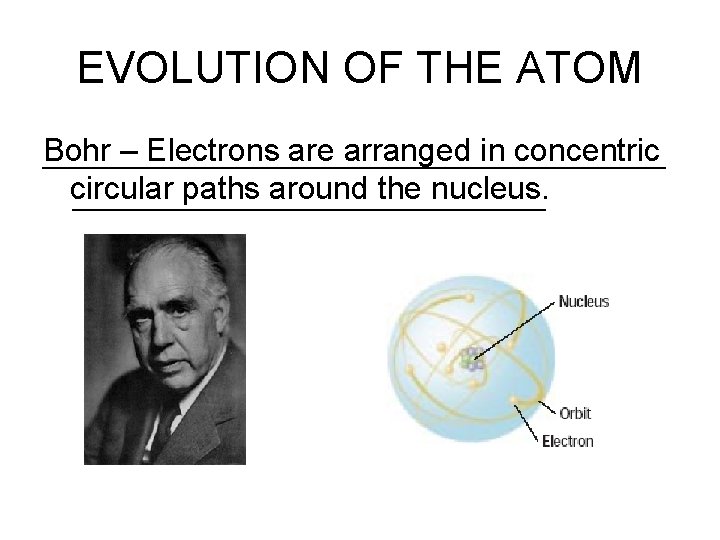
EVOLUTION OF THE ATOM Bohr – Electrons are arranged in concentric circular paths around the nucleus.

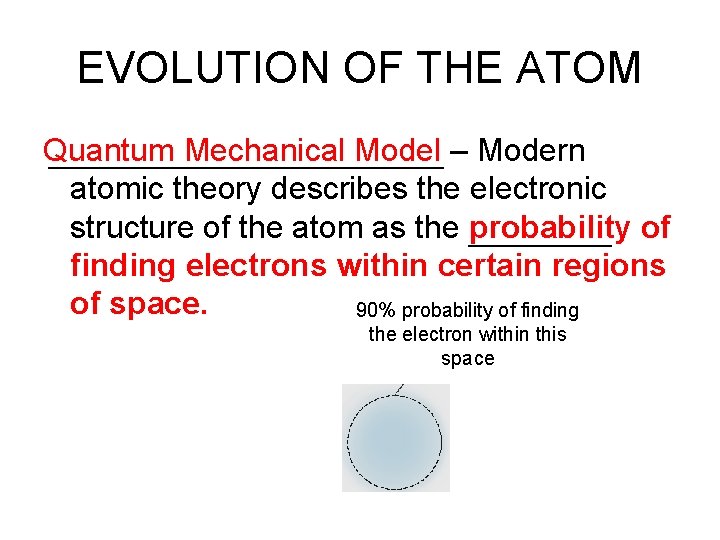
EVOLUTION OF THE ATOM Quantum Mechanical Model – Modern atomic theory describes the electronic structure of the atom as the probability of finding electrons within certain regions of space. 90% probability of finding the electron within this space

WHERE ARE THE ELECTRONS? The quantum mechanical model is a theoretical mathematical approach to the study of atomic and molecular structure – a very complex theory! So let’s not go there. Instead we will learn some of the basic concepts using a visual that we can all relate to: an apartment building. Vs.
![ELECTRON APARTMENTS The apartment building has different floors principal energy level different apartments on ELECTRON APARTMENTS The apartment building has different floors [principal energy level], different apartments on](https://slidetodoc.com/presentation_image/14344375854d0d1c0e6504d647fb3ddc/image-10.jpg)
ELECTRON APARTMENTS The apartment building has different floors [principal energy level], different apartments on each floor [sublevel], and rooms [orbitals] within each apartment.

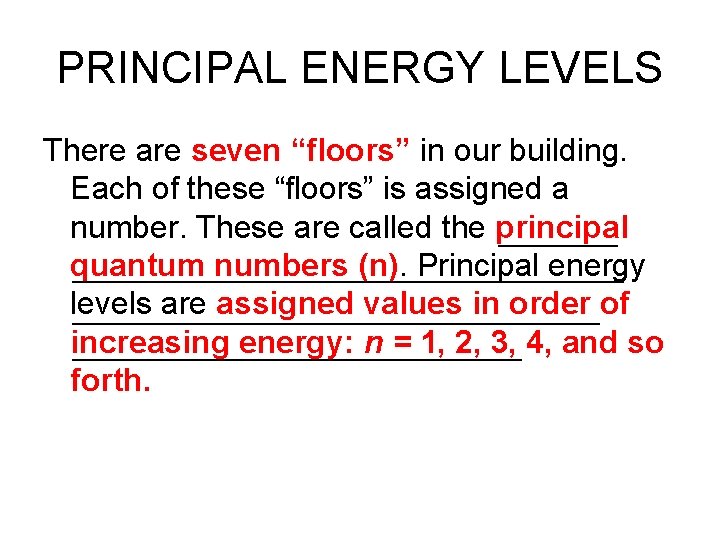
PRINCIPAL ENERGY LEVELS There are seven “floors” in our building. Each of these “floors” is assigned a number. These are called the principal quantum numbers (n). Principal energy levels are assigned values in order of increasing energy: n = 1, 2, 3, 4, and so forth.

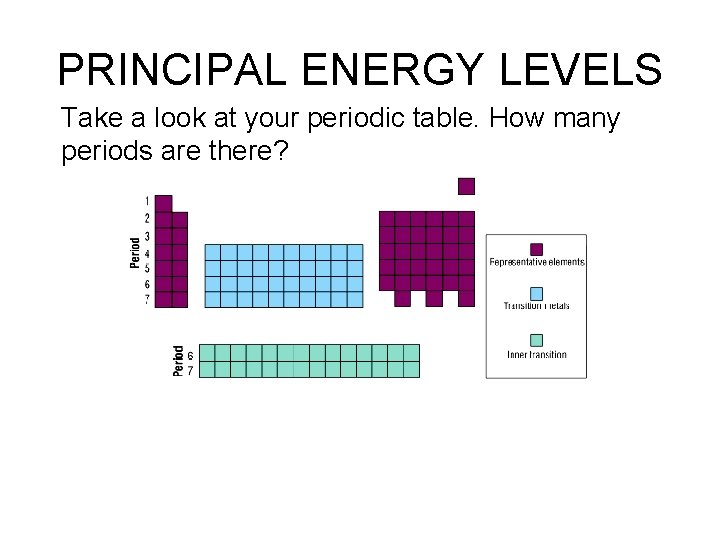
PRINCIPAL ENERGY LEVELS Take a look at your periodic table. How many periods are there?

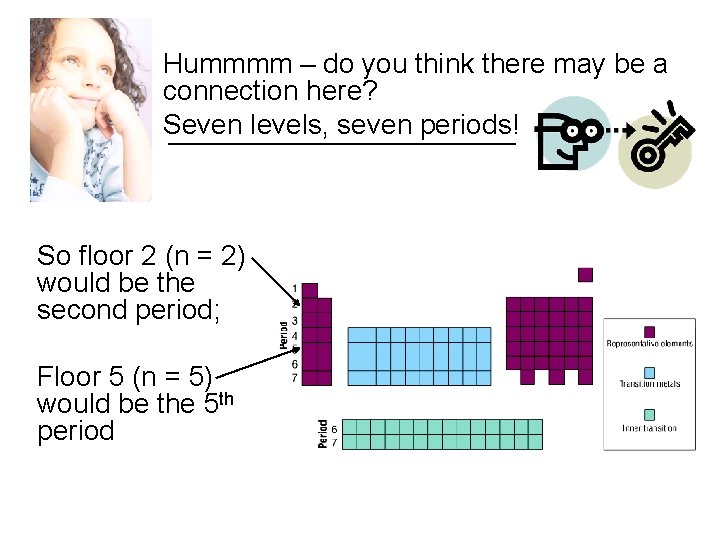
Hummmm – do you think there may be a connection here? Seven levels, seven periods! So floor 2 (n = 2) would be the second period; Floor 5 (n = 5) would be the 5 th period

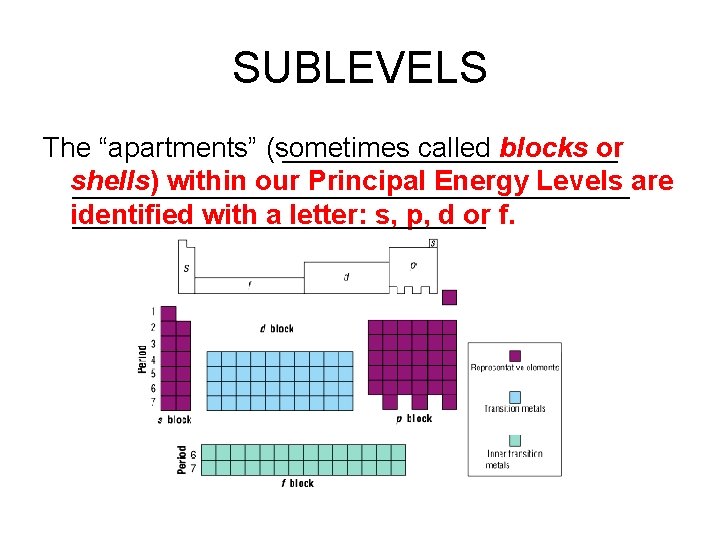
SUBLEVELS The “apartments” (sometimes called blocks or shells) within our Principal Energy Levels are identified with a letter: s, p, d or f.

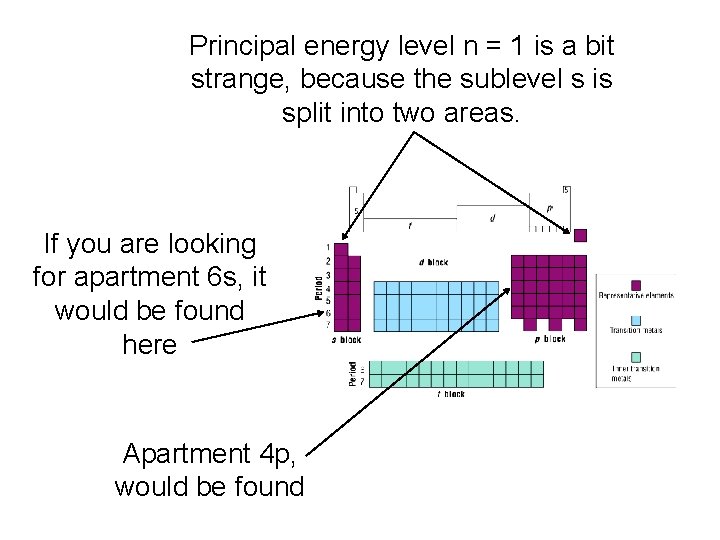
Principal energy level n = 1 is a bit strange, because the sublevel s is split into two areas. If you are looking for apartment 6 s, it would be found here Apartment 4 p, would be found

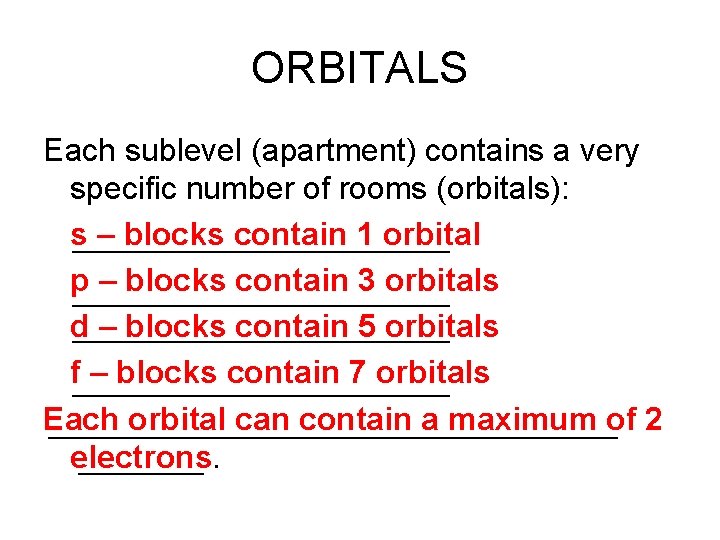
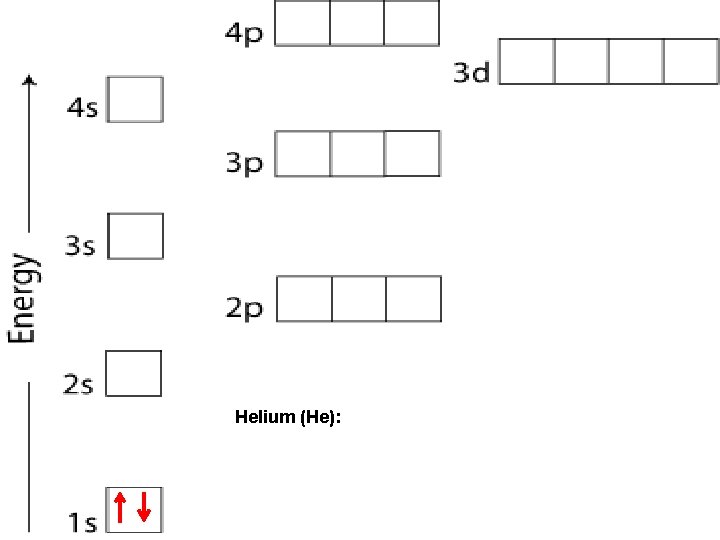
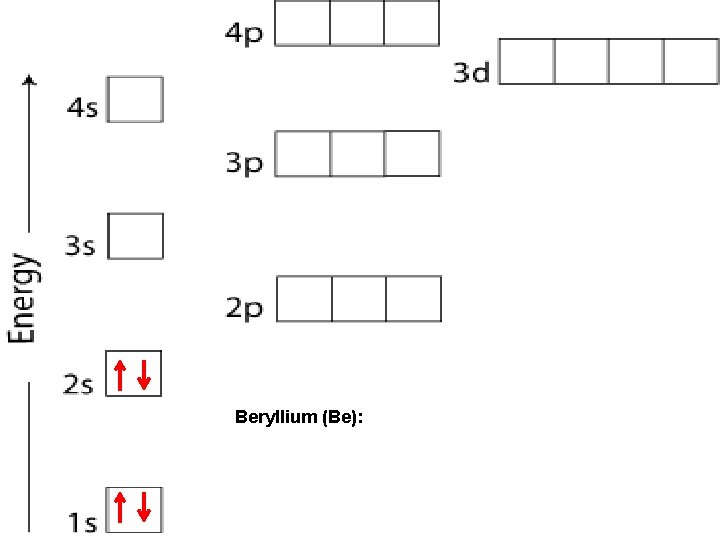
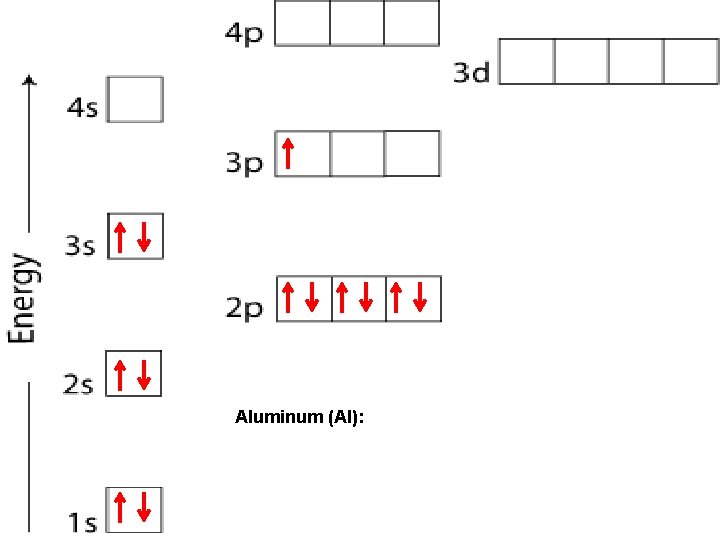
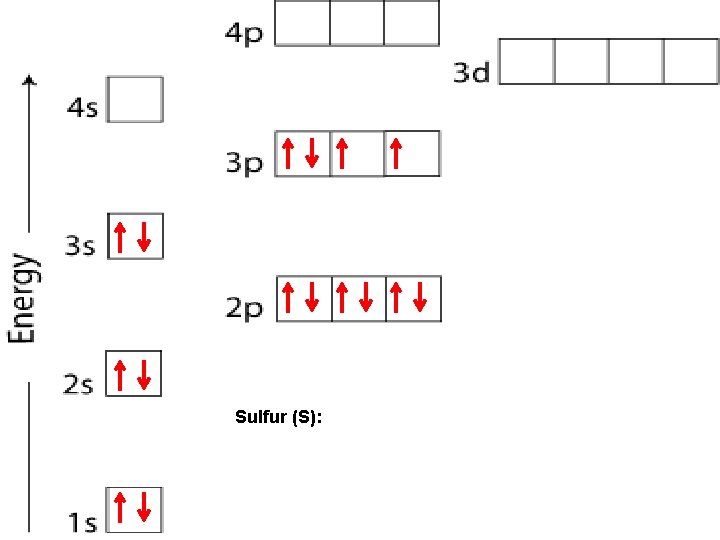
ORBITALS Each sublevel (apartment) contains a very specific number of rooms (orbitals): s – blocks contain 1 orbital p – blocks contain 3 orbitals d – blocks contain 5 orbitals f – blocks contain 7 orbitals Each orbital can contain a maximum of 2 electrons.

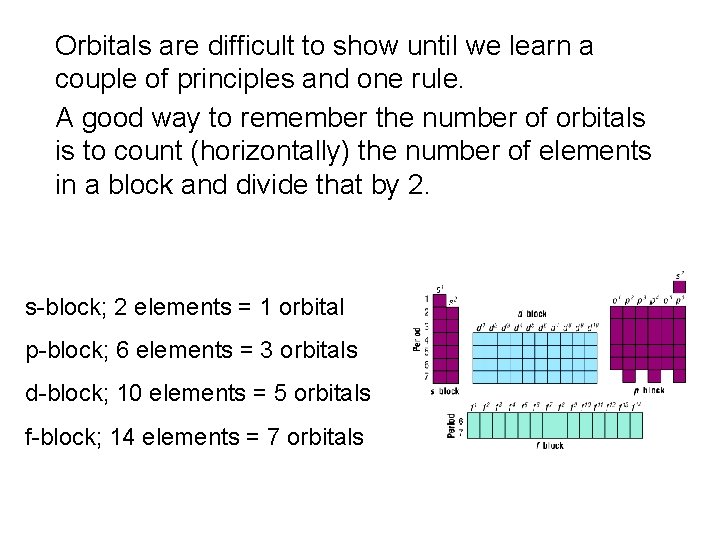
Orbitals are difficult to show until we learn a couple of principles and one rule. A good way to remember the number of orbitals is to count (horizontally) the number of elements in a block and divide that by 2. s-block; 2 elements = 1 orbital p-block; 6 elements = 3 orbitals d-block; 10 elements = 5 orbitals f-block; 14 elements = 7 orbitals

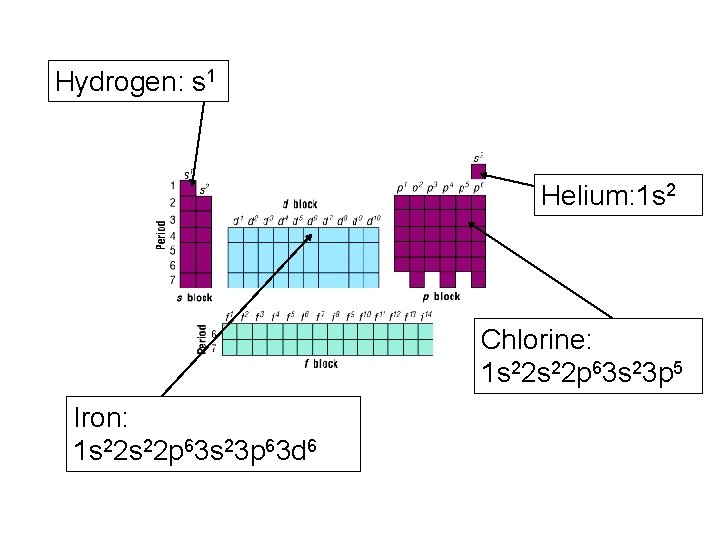
ELECTRON CONFIGURATION The electron configuration actually gives us the location of any element on the periodic table. We simply have to be able to count as we fill in boxes! The way we read the configuration is to account for every electron in the atom – time to remember that as elements progress across the periodic table, the number of protons and electrons increase by one. A little practice is all it takes.

Hydrogen: s 1 Helium: 1 s 2 Chlorine: 1 s 22 p 63 s 23 p 5 Iron: 1 s 22 p 63 s 23 p 63 d 6

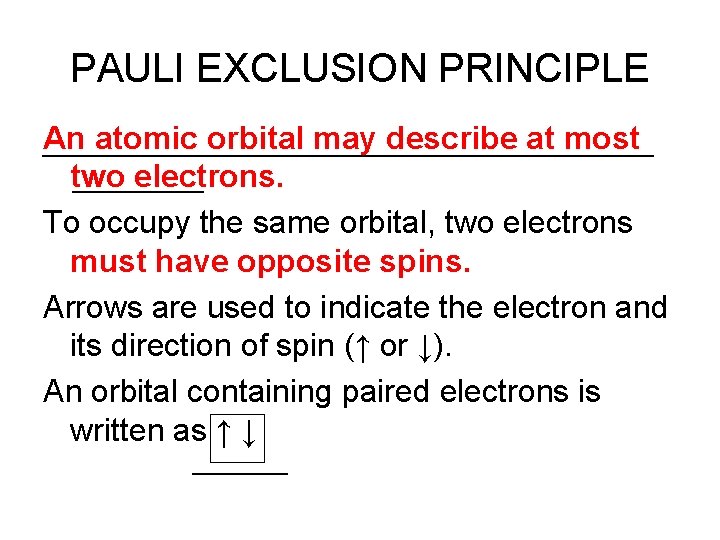
PAULI EXCLUSION PRINCIPLE An atomic orbital may describe at most two electrons. To occupy the same orbital, two electrons must have opposite spins. Arrows are used to indicate the electron and its direction of spin (↑ or ↓). An orbital containing paired electrons is written as ↑ ↓

HUND’S RULE When electrons occupy orbitals of equal energy, one electron enters each orbital until all the orbitals contain one electron with parallel spins. p orbitals When the 4 th electron is needed, it will occupy the first orbital and so on - - -

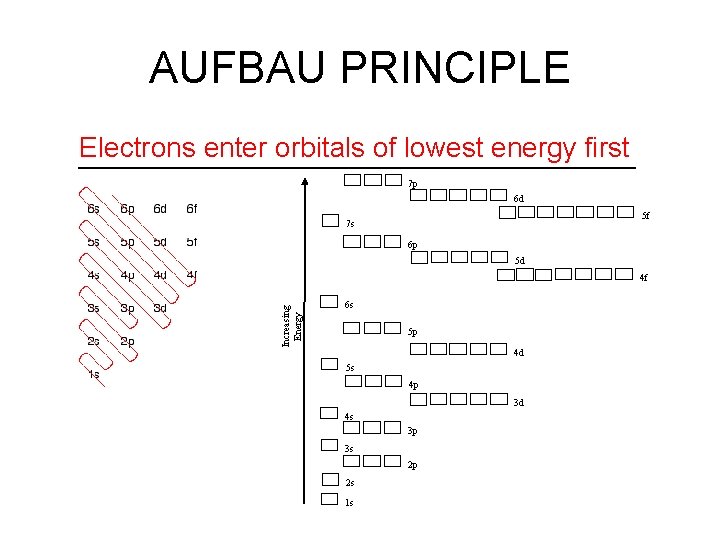
AUFBAU PRINCIPLE Electrons enter orbitals of lowest energy first 7 p 6 d 5 f 7 s 6 p 5 d Increasing Energy 4 f 6 s 5 p 4 d 5 s 4 p 3 d 4 s 3 p 3 s 2 p 2 s 1 s

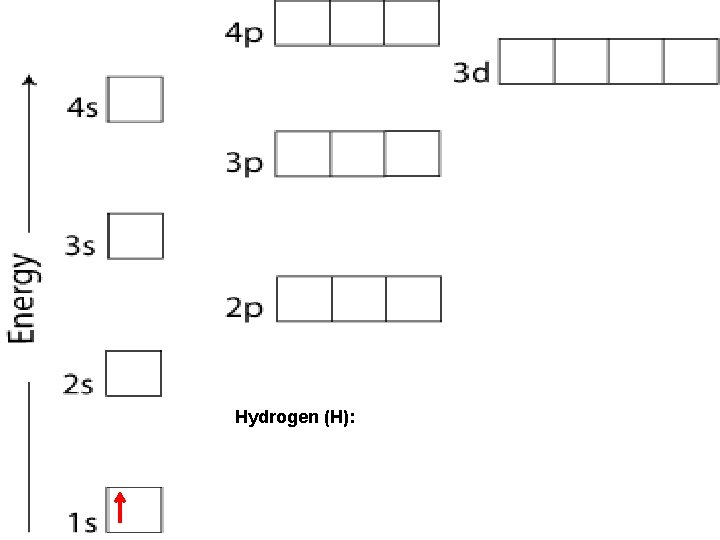
Hydrogen (H):

Helium (He):

Beryllium (Be):

Aluminum (Al):

Sulfur (S):