Chapter 8 Electron Configurations and Periodicity Contents and










































- Slides: 72

Chapter 8 Electron Configurations and Periodicity

Contents and Concepts Electronic Structure of Atoms In the previous chapter, you learned that we characterize an atomic orbital by four quantum numbers: n, l, ml, and ms. In the first section, we look further at electron spin; then we discuss how electrons are distributed among the possible orbitals of an atom. 1. Electron Spin and the Pauli Exclusion Principle 2. Building-Up Principle and the Periodic Table 3. Writing Electron Configurations Using the Periodic Table 4. Orbital Diagrams of Atoms; Hund’s Rule 8|2

Periodicity of the Elements You learned how the periodic table can be explained by the periodicity of the ground-state configurations of the elements. Now we will look at various aspects of the periodicity of the elements. 5. Mendeleev’s Predictions from the Periodic Table 6. Some Periodic Properties 7. Periodicity in the Main-Group Elements 8|3

Learning Objectives Electronic Structure of Atoms 1. Electron Spin and the Pauli Exclusion Principle a. Define electron configuration and orbital diagram. b. State the Pauli exclusion principle. c. Apply the Pauli exclusion principle. 8|4

2. Building-Up Principle and the Periodic Table a. Define building-up principle. b. Define noble-gas core, pseudo-noble-gas core, and valence electron. c. Define main-group element and (d-block and f-block) transition element. 3. Writing Electron Configurations Using the Periodic Table a. Determine the configuration of an atom using the building-up principle. b. Determine the configuration of an atom using the period and group number. 8|5

4. Orbital Diagrams of Atoms; Hund’s Rule a. State Hund’s rule. b. Apply Hund’s rule. c. Define paramagnetic substance and diamagnetic substance. Periodicity of the Elements 5. Mendeleev’s Predictions from the Periodic Table a. Describe how Mendeleev predicted the properties of undiscovered elements. 8|6

6. Some Periodic Properties a. State the periodic law. b. State the general periodic trends in size of atomic radii. c. Define effective nuclear charge. d. Determine relative atomic sizes from periodic trends. e. State the general periodic trends in ionization energy. f. Define first ionization energy. g. Determine relative ionization energies from periodic trends. 8|7

h. Define electron affinity. i. State the broad general trend in electron affinity across any period. 7. Periodicity in the Main-Group Elements a. Define basic oxide, acidic oxide, and amphoteric oxide. b. State the main group corresponding to an alkali metal, an alkaline earth metal, a chalcogen, a halogen, and a noble gas. c. Describe the change in metallic/nonmetallic character (or reactivities) in going through any main group of elements. 8|8

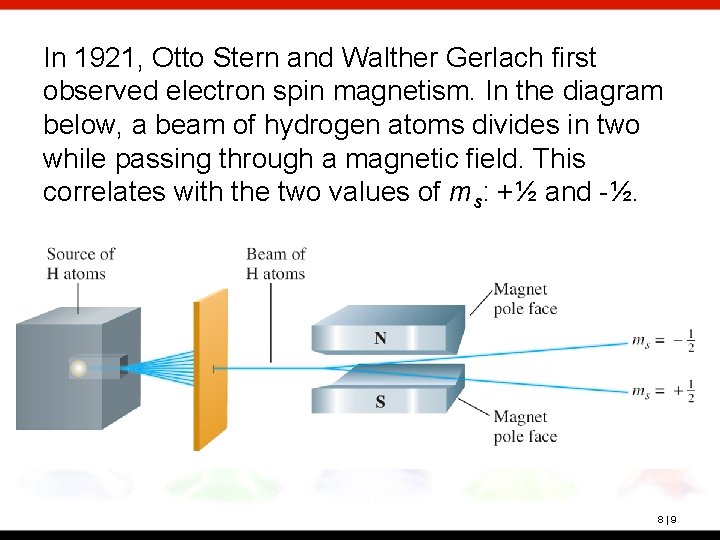
In 1921, Otto Stern and Walther Gerlach first observed electron spin magnetism. In the diagram below, a beam of hydrogen atoms divides in two while passing through a magnetic field. This correlates with the two values of ms: +½ and -½. 8|9

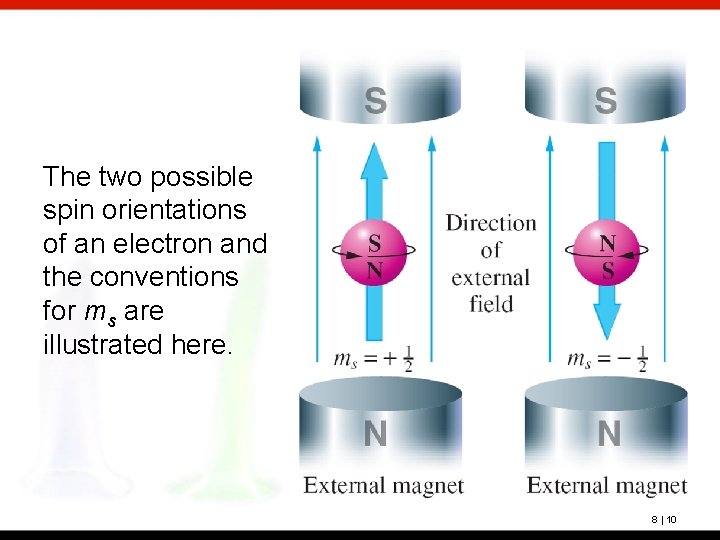
The two possible spin orientations of an electron and the conventions for ms are illustrated here. 8 | 10

An electron configuration of an atom is a particular distribution of electrons among available subshells. An orbital diagram of an atom shows how the orbitals of a subshell are occupied by electrons. Orbitals are represented with a circle; electrons are represented with arrows up for ms= +½ or down for ms= -½. 8 | 11

The Pauli exclusion principle summarizes experimental observations that no two electrons in one atom can have the same four quantum numbers. That means that within one orbital, electrons must have opposite spin. It also means that one orbital can hold a maximum of two electrons (with opposite spin). 8 | 12

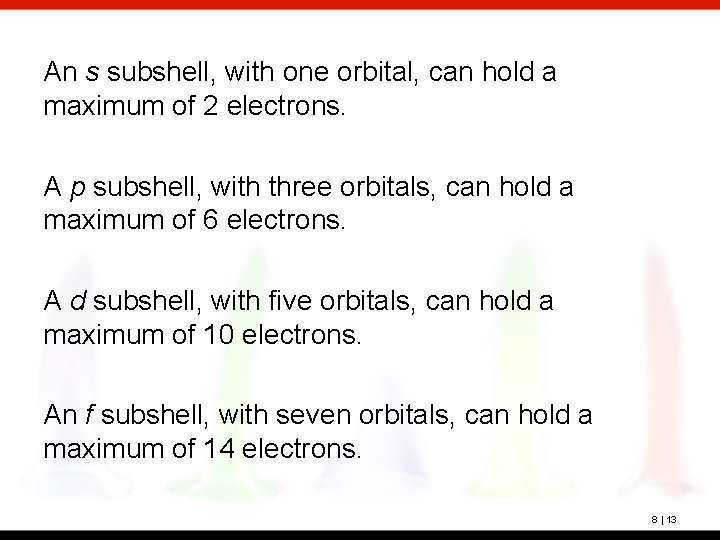
An s subshell, with one orbital, can hold a maximum of 2 electrons. A p subshell, with three orbitals, can hold a maximum of 6 electrons. A d subshell, with five orbitals, can hold a maximum of 10 electrons. An f subshell, with seven orbitals, can hold a maximum of 14 electrons. 8 | 13

The lowest-energy configuration of an atom is called its ground state. Any other configuration represents an excited state. 8 | 14

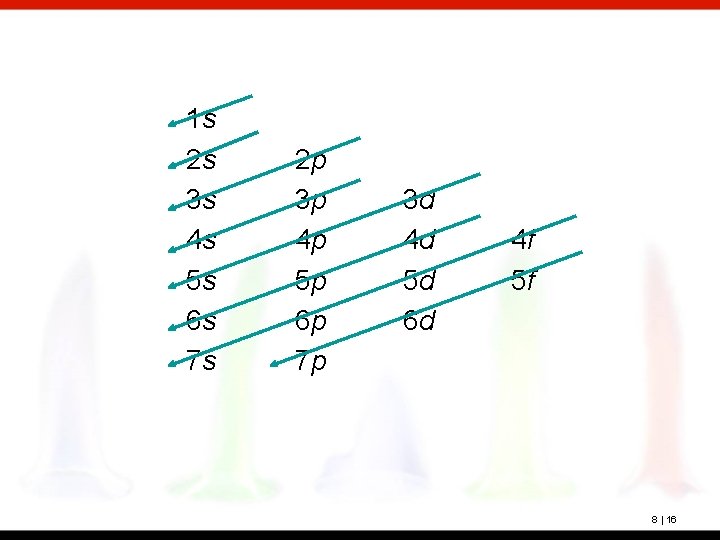
The building-up principle (or aufbau principle) is a scheme used to reproduce the ground-state electron configurations by successively filling subshells with electrons in a specific order (the building-up order). This order generally corresponds to filling the orbitals from lowest to highest energy. Note that these energies are the total energy of the atom rather than the energy of the subshells alone. 8 | 15

1 s 2 s 3 s 4 s 5 s 6 s 7 s 2 p 3 p 4 p 5 p 6 p 7 p 3 d 4 d 5 d 6 d 4 f 5 f 8 | 16

This results in the following order: 1 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, 5 s, 4 d, 5 p, 6 s, 4 f, 5 d, 6 p, 7 s, 5 f, 6 d, 7 p 8 | 17

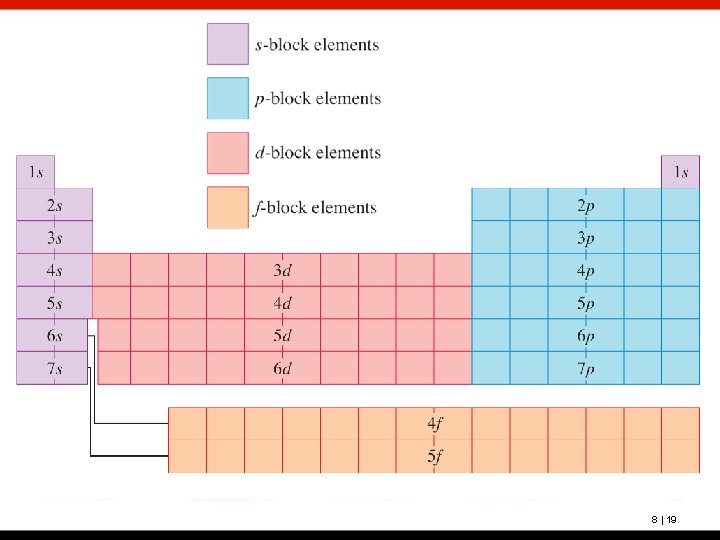
Another way to learn the building-up order is to correlate each subshell with a position on the periodic table. The principal quantum number, n, correlates with the period number. Groups IA and IIA correspond to the s subshell; Groups IIIA through VIIIA correspond to the p subshell; the “B” groups correspond to the d subshell; and the bottom two rows correspond to the f subshell. This is shown on the next slide. 8 | 18

8 | 19

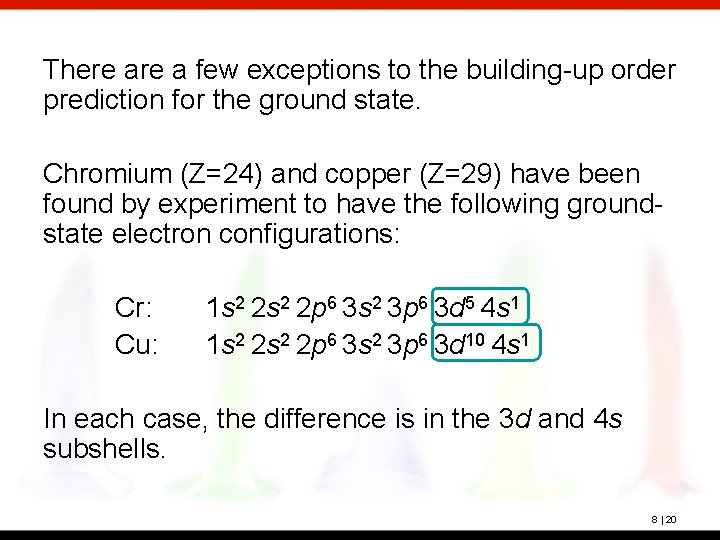
There a few exceptions to the building-up order prediction for the ground state. Chromium (Z=24) and copper (Z=29) have been found by experiment to have the following groundstate electron configurations: Cr: Cu: 1 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 1 In each case, the difference is in the 3 d and 4 s subshells. 8 | 20

There are several terms describing electron configurations that are important. The complete electron configuration shows every subshell explicitly. Br: 1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 5 8 | 21

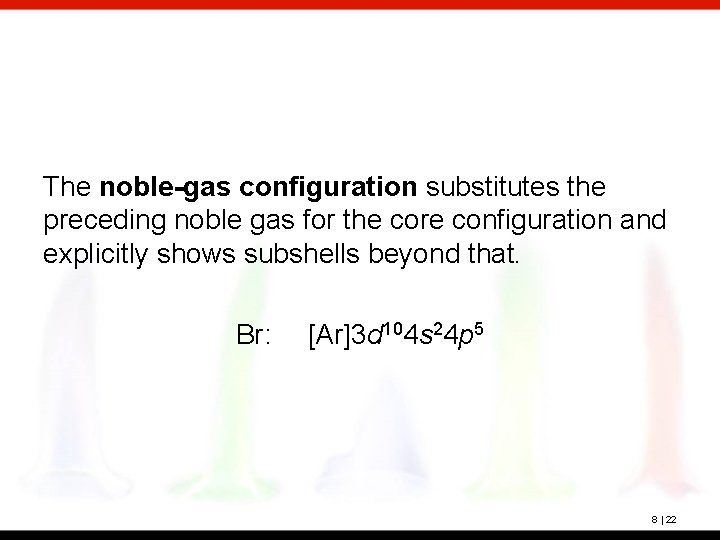
The noble-gas configuration substitutes the preceding noble gas for the core configuration and explicitly shows subshells beyond that. Br: [Ar]3 d 104 s 24 p 5 8 | 22

The pseudo-noble-gas core includes the noblegas subshells and the filled inner, (n – 1), d subshell. For bromine, the pseudo-noble-gas core is [Ar]3 d 10 8 | 23

The valence configuration consists of the electrons outside the noble-gas or pseudo-noblegas core. Br: 4 s 24 p 5 8 | 24

For main-group (representative) elements, an s or a p subshell is being filled. For d-block transition elements, a d subshell is being filled. For f-block transition elements, an f subshell is being filled. 8 | 25

For main-group elements, the valence configuration is in the form ns. Anp. B The sum of A and B is equal to the group number. So, for an element in Group VA of the third period, the valence configuration is 3 s 23 p 3 8 | 26

? Write the complete electron configuration of the arsenic atom, As, using the building-up principle. For arsenic, As, Z = 33. 1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 3 8 | 27

? What are the electron configurations for the valence electrons of arsenic and zinc? Arsenic is in period 4, Group VA. Its valence configuration is 4 s 24 p 3. Zinc, Z = 30, is a transition metal in the first transition series. Its noble-gas core is Ar, Z = 18. Its valence configuration is 4 s 23 d 10. 8 | 28

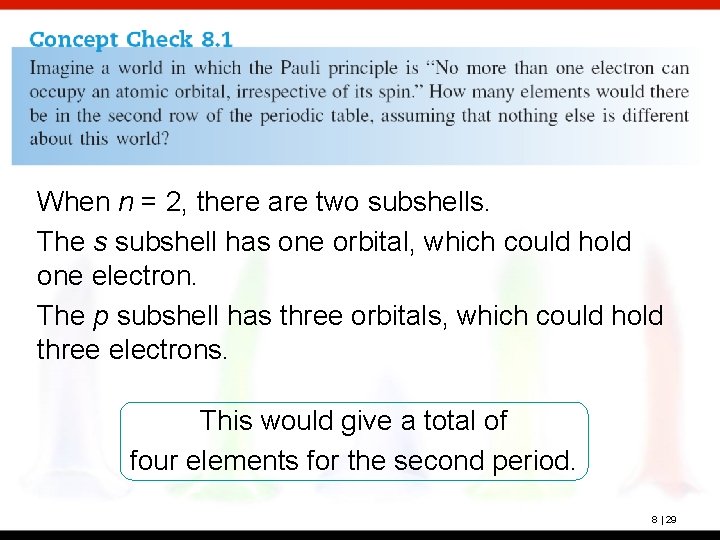
When n = 2, there are two subshells. The s subshell has one orbital, which could hold one electron. The p subshell has three orbitals, which could hold three electrons. This would give a total of four elements for the second period. 8 | 29

In 1927, Friedrich Hund discovered, by experiment, a rule for determining the lowest-energy configuration of electrons in orbitals of a subshell. Hund’s rule states that the lowest-energy arrangement of electrons in a subshell is obtained by putting electrons into separate orbitals of the subshell with the same spin before pairing electrons. 8 | 30

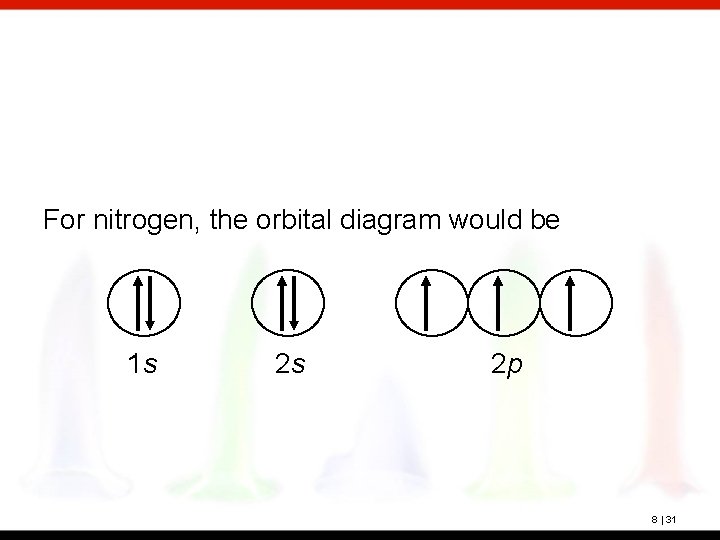
For nitrogen, the orbital diagram would be 1 s 2 s 2 p 8 | 31

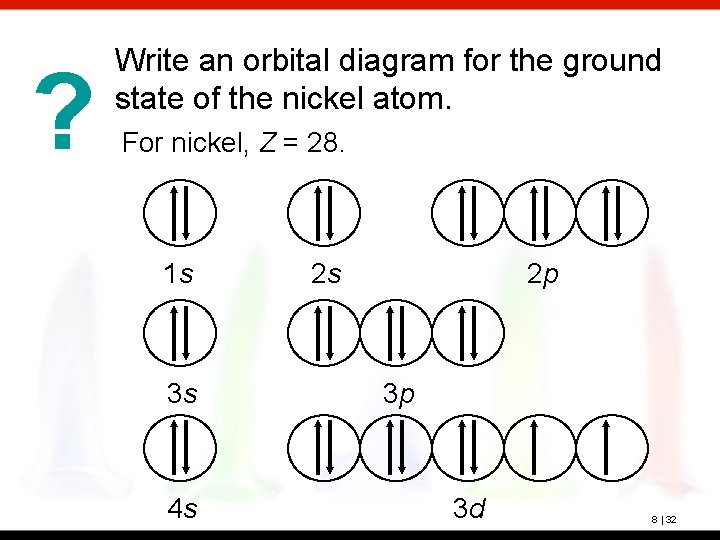
? Write an orbital diagram for the ground state of the nickel atom. For nickel, Z = 28. 1 s 3 s 4 s 2 s 2 p 3 p 3 d 8 | 32

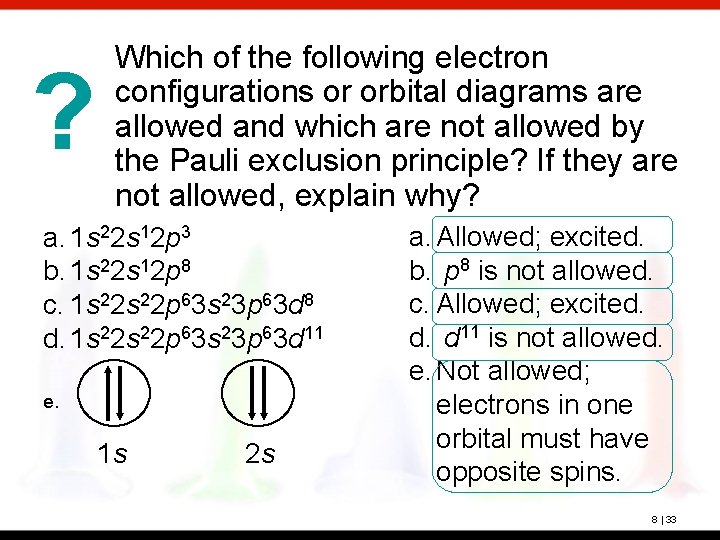
? Which of the following electron configurations or orbital diagrams are allowed and which are not allowed by the Pauli exclusion principle? If they are not allowed, explain why? a. 1 s 22 s 12 p 3 b. 1 s 22 s 12 p 8 c. 1 s 22 p 63 s 23 p 63 d 8 d. 1 s 22 p 63 s 23 p 63 d 11 e. 1 s 2 s a. Allowed; excited. b. p 8 is not allowed. c. Allowed; excited. d. d 11 is not allowed. e. Not allowed; electrons in one orbital must have opposite spins. 8 | 33

Magnetic Properties of Atoms Although an electron behaves like a tiny magnet, two electrons that are opposite in spin cancel each other. Only atoms with unpaired electrons exhibit magnetic susceptibility. This allows us to classify atoms based on their behavior in a magnetic field. 8 | 34

A paramagnetic substance is one that is weakly attracted by a magnetic field, usually as the result of unpaired electrons. A diamagnetic substance is not attracted by a magnetic field generally because it has only paired electrons. 8 | 35

You learned how the organization of the periodic table can be explained by the periodicity of the ground-state configurations of the elements. Now we will look at various aspects of the periodicity of the elements. 8 | 36

Mendeleev’s periodic table generally organized elements by increasing atomic mass and with similar properties in columns. In some places, there were missing elements whose properties he predicted. When gallium, scandium, and germanium were isolated and characterized, their properties were almost identical to those predicted by Mendeleev for eka-aluminum, eka-boron, and eka-silicon, respectively. 8 | 37

Periodic law states that when the elements are arranged by atomic number, their physical and chemical properties vary periodically. We will look in more detail at three periodic properties: atomic radius, ionization energy, and electron affinity. 8 | 38

Atomic Radius While an atom does not have a definite size, we can define it in terms of covalent radii (the radius in covalent compounds). 8 | 39

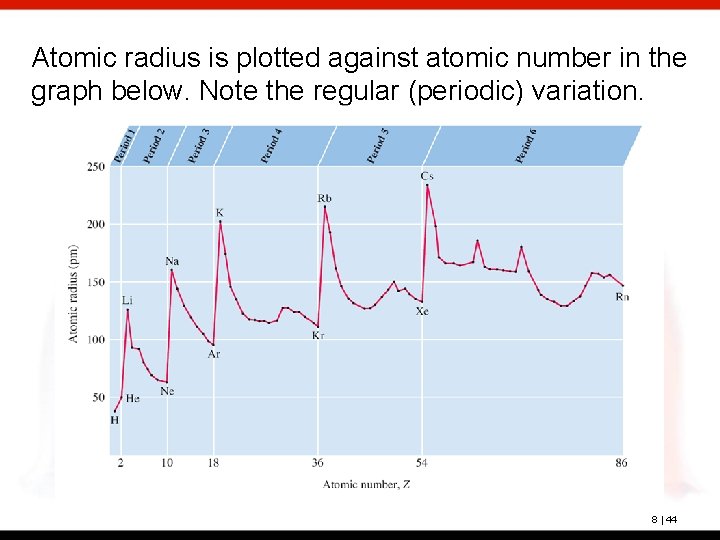
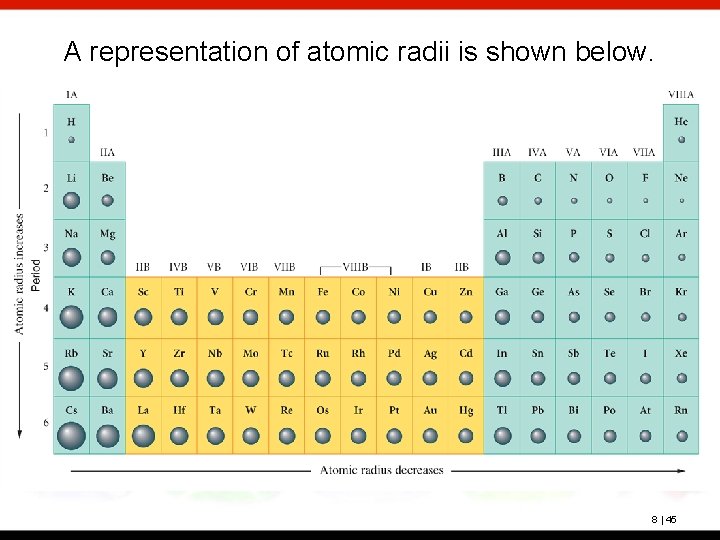
Trends Within each group (vertical column), the atomic radius increases with the period number. This trend is explained by the fact that each successive shell is larger than the previous shell. 8 | 40

Within each period (horizontal row), the atomic radius tends to decrease with increasing atomic number (nuclear charge). 8 | 41

Effective Nuclear Charge Effective nuclear charge is the positive charge that an electron experiences from the nucleus. It is equal to the nuclear charge, but is reduced by shielding or screening from any intervening electron distribution (inner shell electrons). 8 | 42

Effective nuclear charge increases across a period. Because the shell number (n) is the same across a period, each successive atom experiences a stronger nuclear charge. As a result, the atomic size decreases across a period. 8 | 43

Atomic radius is plotted against atomic number in the graph below. Note the regular (periodic) variation. 8 | 44

A representation of atomic radii is shown below. 8 | 45

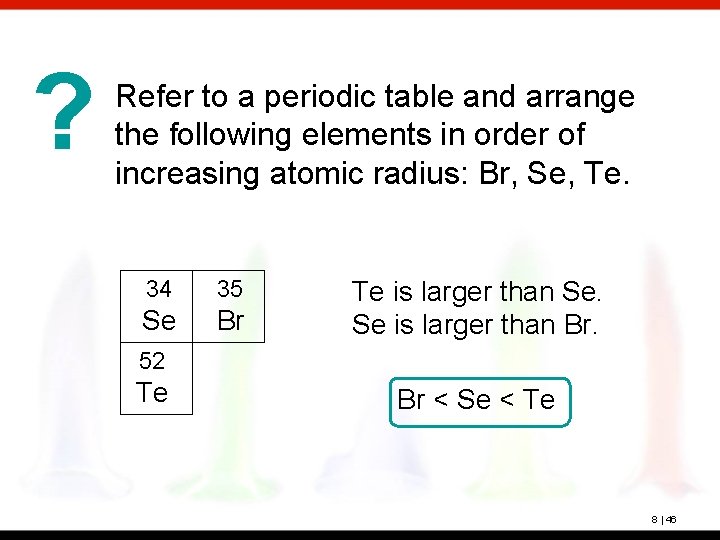
? Refer to a periodic table and arrange the following elements in order of increasing atomic radius: Br, Se, Te. 34 35 Se Br Te is larger than Se. Se is larger than Br. 52 Te Br < Se < Te 8 | 46

First Ionization Energy (first ionization potential) The minimum energy needed to remove the highest-energy (outermost) electron from a neutral atom in the gaseous state, thereby forming a positive ion 8 | 47

Trends Going down a group, first ionization energy decreases. This trend is explained by understanding that the smaller an atom, the harder it is to remove an electron, so the larger the ionization energy. 8 | 48

Generally, ionization energy increases with atomic number. Ionization energy is proportional to the effective nuclear charge divided by the average distance between the electron and the nucleus. Because the distance between the electron and the nucleus is inversely proportional to the effective nuclear charge, ionization energy is inversely proportional to the square of the effective nuclear charge. 8 | 49

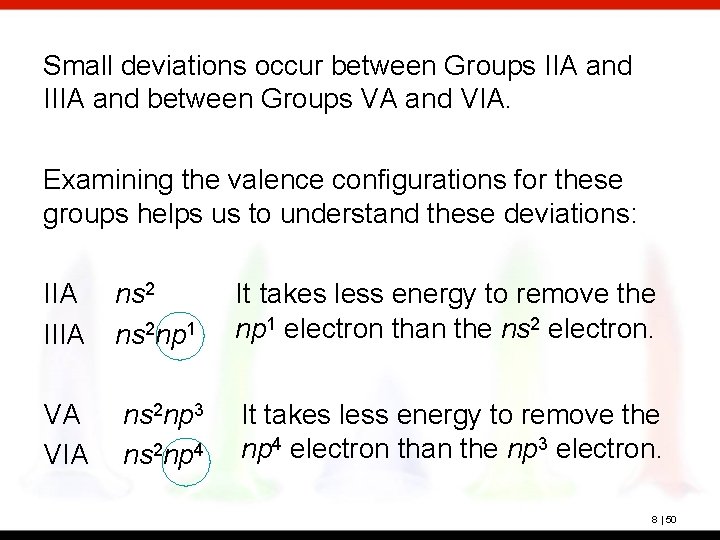
Small deviations occur between Groups IIA and IIIA and between Groups VA and VIA. Examining the valence configurations for these groups helps us to understand these deviations: IIA IIIA ns 2 np 1 It takes less energy to remove the np 1 electron than the ns 2 electron. VA VIA ns 2 np 3 ns 2 np 4 It takes less energy to remove the np 4 electron than the np 3 electron. 8 | 50

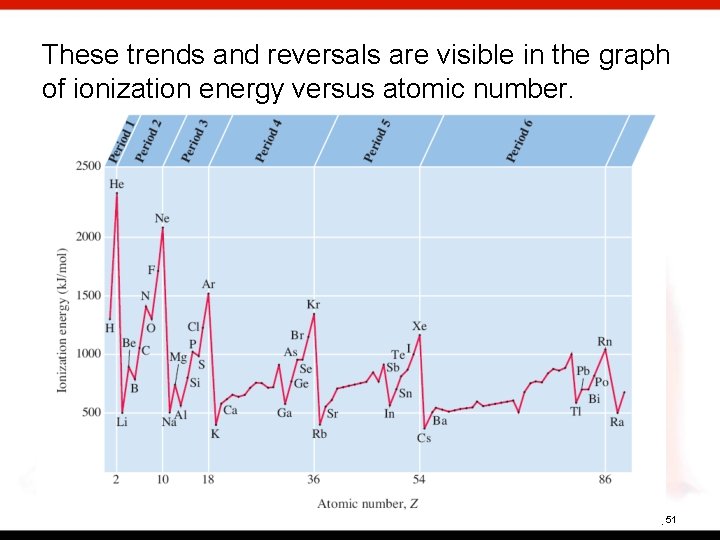
These trends and reversals are visible in the graph of ionization energy versus atomic number. 8 | 51

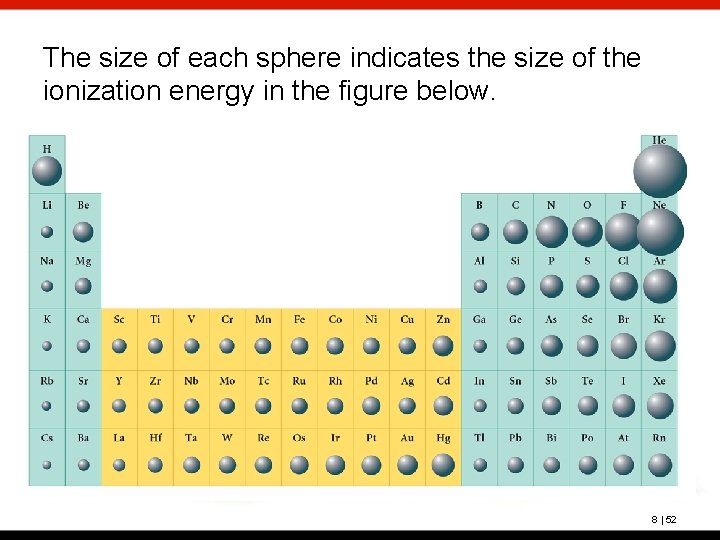
The size of each sphere indicates the size of the ionization energy in the figure below. 8 | 52

Electrons can be successively removed from an atom. Each successive ionization energy increases, because the electron is removed from a positive ion of increasing charge. A dramatic increase occurs when the first electron from the noble-gas core is removed. 8 | 53

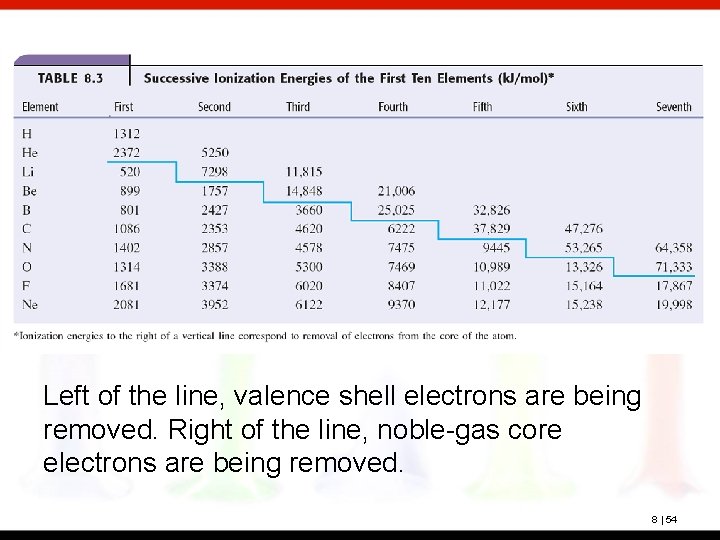
Left of the line, valence shell electrons are being removed. Right of the line, noble-gas core electrons are being removed. 8 | 54

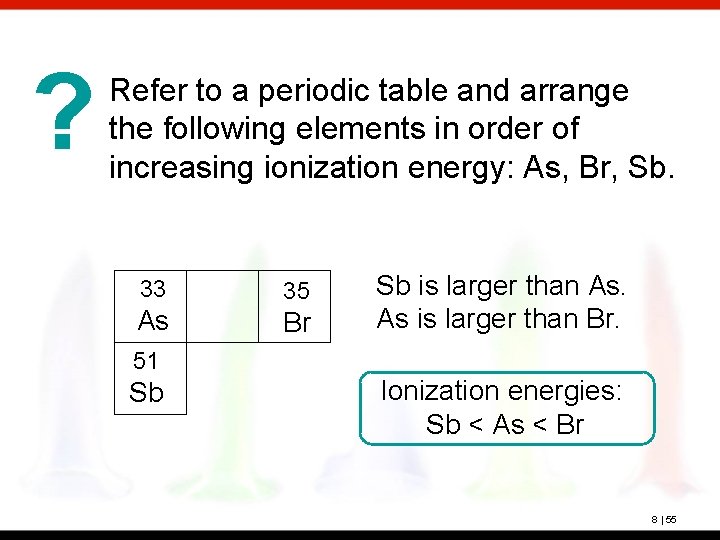
? Refer to a periodic table and arrange the following elements in order of increasing ionization energy: As, Br, Sb. 33 As 35 Br Sb is larger than As. As is larger than Br. 51 Sb Ionization energies: Sb < As < Br 8 | 55

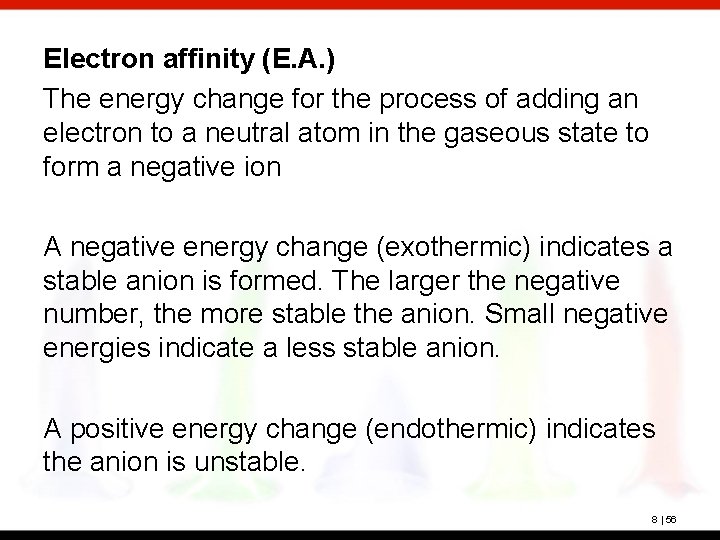
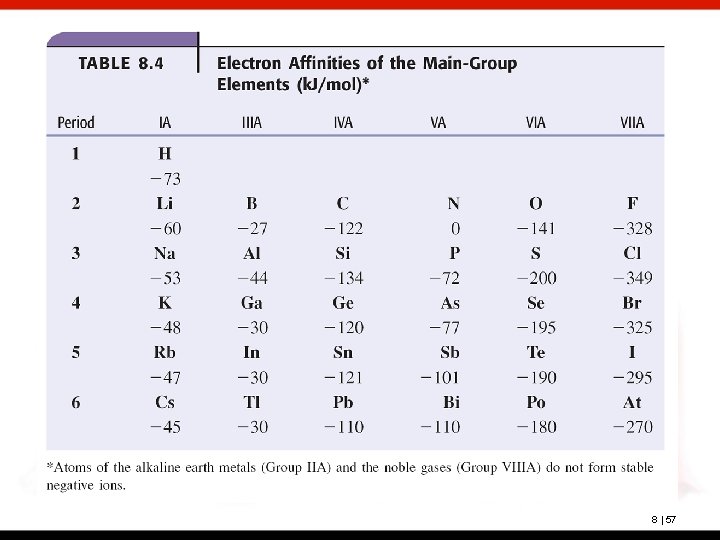
Electron affinity (E. A. ) The energy change for the process of adding an electron to a neutral atom in the gaseous state to form a negative ion A negative energy change (exothermic) indicates a stable anion is formed. The larger the negative number, the more stable the anion. Small negative energies indicate a less stable anion. A positive energy change (endothermic) indicates the anion is unstable. 8 | 56

8 | 57

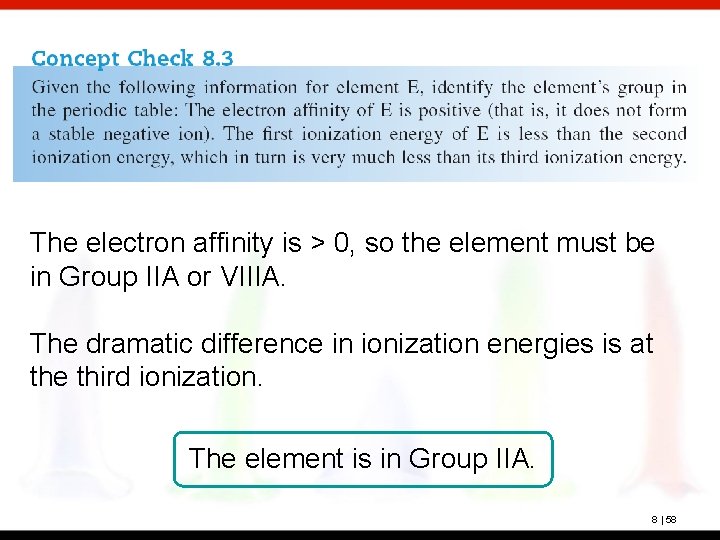
The electron affinity is > 0, so the element must be in Group IIA or VIIIA. The dramatic difference in ionization energies is at the third ionization. The element is in Group IIA. 8 | 58

Broadly speaking, the trend is toward more negative electron affinities going from left to right in a period. Let’s explore the periodic table by group. 8 | 59

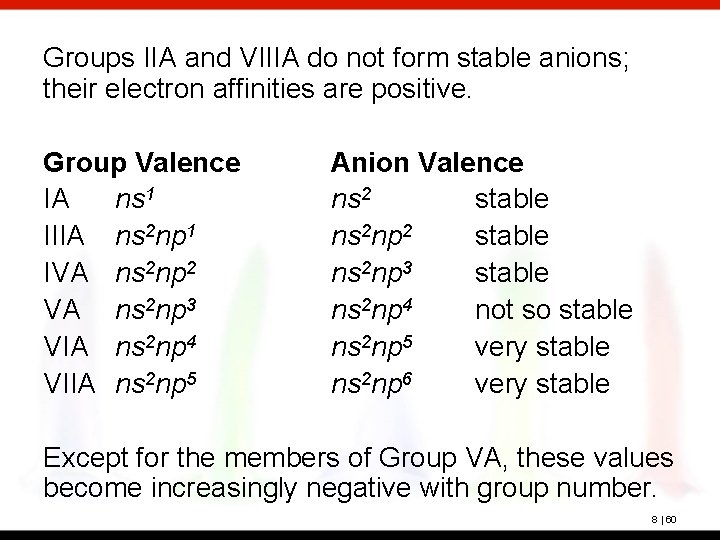
Groups IIA and VIIIA do not form stable anions; their electron affinities are positive. Group Valence IA ns 1 IIIA ns 2 np 1 IVA ns 2 np 2 VA ns 2 np 3 VIA ns 2 np 4 VIIA ns 2 np 5 Anion Valence ns 2 stable ns 2 np 3 stable ns 2 np 4 not so stable ns 2 np 5 very stable ns 2 np 6 very stable Except for the members of Group VA, these values become increasingly negative with group number. 8 | 60

Metallic Character Elements with low ionization energies tend to be metals. Those with high ionization energies tend to be nonmetals. This can vary within a group as well as within a period. 8 | 61

Oxides A basic oxide reacts with acids. Most metal oxides are basic. If soluble, their water solutions are basic. An acidic oxide reacts with bases. Most nonmetal oxides are acidic. If soluble, their water solutions are acidic. An amphoteric oxide reacts with both acids and bases. 8 | 62

Group IA, Alkali Metals (ns 1) These elements are metals; their reactivity increases down the group. The oxides have the formula M 2 O. Hydrogen is a special case. It usually behaves as a nonmetal, but at very high pressures it can exhibit metallic properties. 8 | 63

Group IIA, Alkaline Earth Metals (ns 2) These elements are metals; their reactivity increases down the group. The oxides have the formula MO. 8 | 64

Group IIIA (ns 2 np 1) Boron is a metalloid; all other members of Group IIIA are metals. The oxide formula is R 2 O 3. B 2 O 3 is acidic; Al 2 O 3 and Ga 2 O 3 are amphoteric; the others are basic. 8 | 65

Group IVA (ns 2 np 2) Carbon is a nonmetal; silicon and germanium are metalloids; tin and lead are metals. The oxide formula is RO 2 and, for carbon and lead, RO. CO 2, Si. O 2, and Ge. O 2 are acidic (decreasingly so). Sn. O 2 and Pb. O 2 are amphoteric. 8 | 66

Some oxides of Group IVA Pb. O (yellow) Si. O 2 (crystalline solid quartz) Pb. O 2 (dark brown) Sn. O 2 (white) 8 | 67

Group VA (ns 2 np 3) Nitrogen and phosphorus are nonmetals; arsenic and antimony are metalloids; bismuth is a metal. The oxide formulas are R 2 O 3 and R 2 O 5, with some molecular formulas being double these. Nitrogen, phosphorus, and arsenic oxides are acidic; antimony oxides are amphoteric; bismuth oxide is basic. 8 | 68

Group VIA, Chalcogens (ns 2 np 4) Oxygen, sulfur, and selenium are nonmetals; tellurium is a metalloid; polonium is a metal. The oxide formulas are RO 2 and RO 3. Sulfur, selenium, and tellurium oxides are acidic except for Te. O 2, which is amphoteric. Po. O 2 is also amphoteric. 8 | 69

Group VIIA, Halogens (ns 2 np 5) These elements are reactive nonmetals, with the general molecular formula being X 2. All isotopes of astatine are radioactive with short half-lives. This element might be expected to be a metalloid. Each halogen forms several acidic oxides that are generally unstable. 8 | 70

Group VIIIA, Noble Gases (ns 2 np 6) These elements are generally unreactive, with only the heavier elements forming unstable compounds. They exist as gaseous atoms. 8 | 71

For R 2 O 5 oxides, R must be in Group VA. R is a metalloid, so R could be As or Sb. The oxide is acidic, so R is arsenic, As. 8 | 72