Unit 7 Atomic Structure and Periodicity EMR Atomic










































































- Slides: 101

Unit 7: Atomic Structure and Periodicity EMR, Atomic Spectra, Quantum Mechanics, Electron Configurations, and Trends Copyright © 2017 Ms. Razz Chem. Class

Unit 7 Topics 7. 1 7. 2 7. 3 7. 4 7. 5 7. 6 7. 7 7. 8 7. 9 7. 10 7. 11 7. 12 7. 13 Electromagnetic Radiation The Nature of Matter The Atomic Spectrum of Hydrogen The Bohr Model The Quantum Mechanical Model of the Atom Quantum Numbers Orbital Shapes and Energies Electron Spin and the Pauli Principle Polyelectronic Atoms The History of the Periodic Table The Aufbau Principle and the Periodic Table Periodic Trends in Atomic Properties The Properties of a Group: The Alkali Metals Copyright © 2017 Ms. Razz Chem. Class

Section 7. 1 - Electromagnetic Radiation (EMR) • One of the ways that energy travels through space. • Examples of EMR: – Light from the sun – Energy used to cook food in a microwave – X rays used by dentists – Radiant heat from a fireplace • All examples of EMR exhibit the same type of wavelike behavior and travel at the speed of light in a vacuum. • Three characteristics: Wavelength, Frequency, Speed Copyright © 2017 Ms. Razz Chem. Class

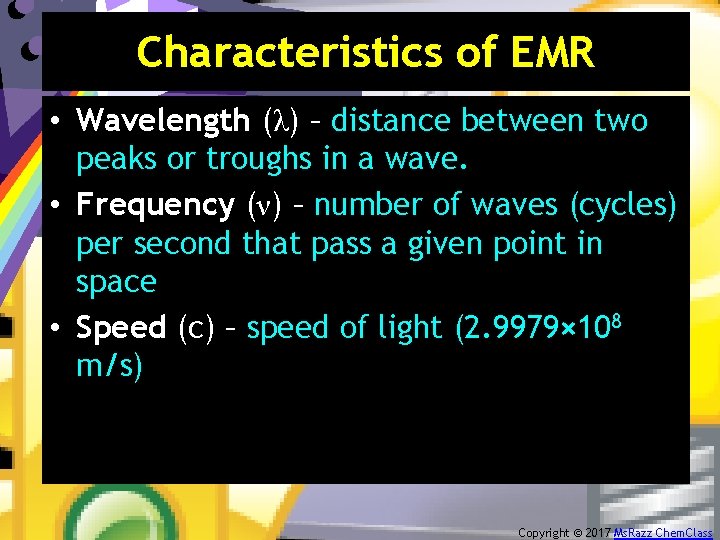
Characteristics of EMR • Wavelength (λ) – distance between two peaks or troughs in a wave. • Frequency (ν) – number of waves (cycles) per second that pass a given point in space • Speed (c) – speed of light (2. 9979× 108 m/s) Copyright © 2017 Ms. Razz Chem. Class

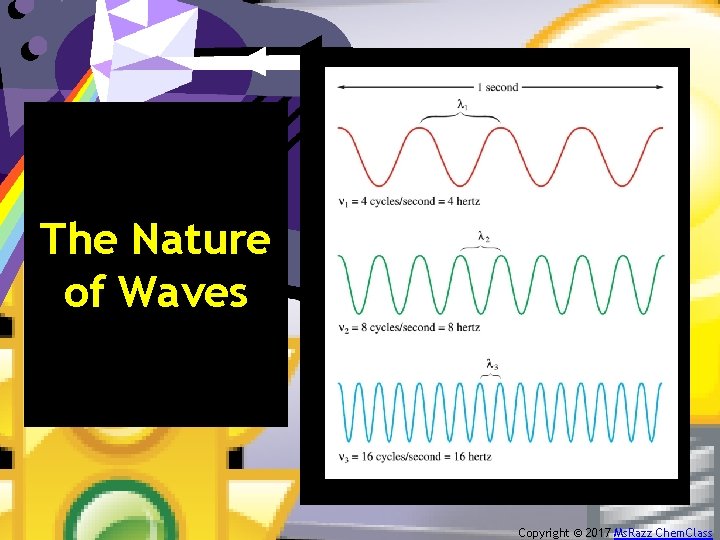
The Nature of Waves Copyright © 2017 Ms. Razz Chem. Class

Characteristics • In the SI system, frequency is measured as 1/s or s-1, which is called Hertz (Hz). • Since all energy that travels by means of EMR, travels at the same speed (c= speed of light), shorter wavelengths must have a higher frequency and longer wavelengths must have a lower frequency. The following equation summarizes this relationship: Copyright © 2017 Ms. Razz Chem. Class

Speed of Waves . c=λν Where, c = speed of light (3. 00 x 108 m/s) λ = wavelength (m or nm) ν = frequency (s-1 or Hz) Copyright © 2017 Ms. Razz Chem. Class

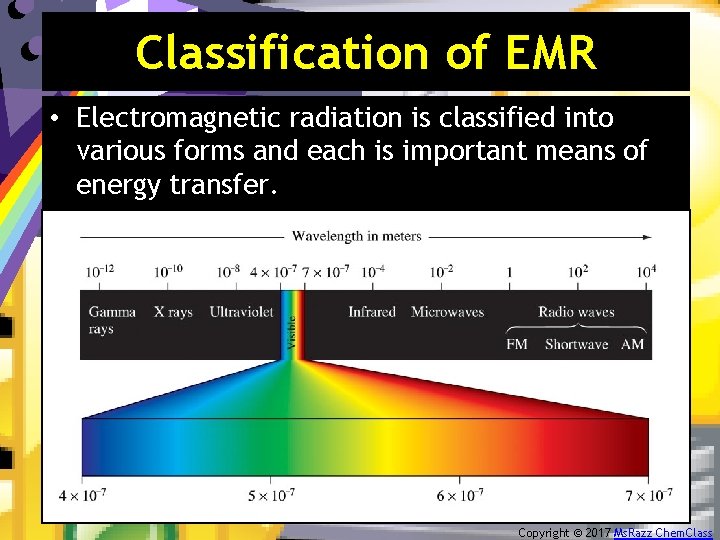
Classification of EMR • Electromagnetic radiation is classified into various forms and each is important means of energy transfer. Copyright © 2017 Ms. Razz Chem. Class

Pickle Light Video Link When a current is applied to a dill pickle, a glowing discharge occurs. The current flows between the forks causing some sodium atoms to reach an excited state. When these atoms relax back to their ground state a yellow glow is visible. Copyright © 2017 Ms. Razz Chem. Class

Example An FM radio station broadcasts at 99. 5 MHz. Calculate the wavelength of the corresponding radio waves. c=λν 99. 5 MHz x 1 x 10 Hz = 9. 95 x 107 Hz 1 MHz 8 m/s) (2. 9979 x 10 λ= = 3. 013 m 7 9. 95 x 10 Hz 6 Copyright © 2017 Ms. Razz Chem. Class

Section 7. 2 – The Nature of Matter At the end of the 19 th century, the idea prevailed that matter and energy were distinct. Matter was thought to consist of particles that had mass and whose position could be specified in space. Energy in the form of light (EMR) was described as a wave that was massless and delocalized. Copyright © 2017 Ms. Razz Chem. Class

Max Planck (1900) In the 20 th century, experimental evidence showed this was incorrect. The first advancement came from German physicist Max Planck who postulated that energy could only be gained or lost in whole-number multiples of quantity hν, where h is a constant called Planck’s constant. h= 6. 626 x 10 -34 J·s Copyright © 2017 Ms. Razz Chem. Class

This proved that: • Energy is quantized and can only occur in discreet units of size hν. • Each packet of energy is called a quantum. ∆E=nhν Where, n= an integer (1, 2, 3…) h= is Planck’s constant ν= is the frequency of the EMR absorbed or emitted Copyright © 2017 Ms. Razz Chem. Class

Example The blue color in fireworks is often achieved by heating copper (I) chloride (Cu. Cl) to about 1200°C. At that temperature the compound emits blue light having a wavelength of 450 nm. What is the increment of energy (that quantum) that is emitted at 4. 50 x 102 nm by Cu. Cl? ν=c/λ 4. 50 x 102 nm x 1 m. = 4. 50 x 10 -7 m 109 nm 8 m/s) (2. 9979 x 10 = 6. 66 x 1014 s-1 ν= ∆E= hν 4. 50 x 10 -7 m ∆E= (6. 626 x 10 -34 J·s) (6. 66 x 1014 s-1) = 4. 41 x 10 -19 J Copyright © 2017 Ms. Razz Chem. Class

Albert Einstein (1905) The next important development in atomic structure came when Albert Einstein proposed that EMR is itself quantized. He suggested that EMR is a stream of “particles” called photons. The energy of each photon can be calculated by: E=hc λ Copyright © 2017 Ms. Razz Chem. Class

Photoelectric Effect Einstein arrived at this conclusion through his analysis of the Photoelectric Effect. In this phenomenon, electrons are emitted from the surface of a metal when light strikes it, but only when the light has a frequency higher than a threshold frequency, v 0. Animation Link: Photoelectric Effect Copyright © 2017 Ms. Razz Chem. Class

Related to this development, Einstein derived his famous equation: E = mc 2 The significance of this equation was that it showed that energy has mass. Copyright © 2017 Ms. Razz Chem. Class

Combining Einstein’s two equations allows us to calculate the apparent mass of one photon using its wavelength. E= hc/λ m= h/λc m= E/c 2 Note: A photon has mass only in a relativistic sense – it has no mass at rest. Copyright © 2017 Ms. Razz Chem. Class

To summarize: • Energy is quantized. It can only occur in discreet units called quantum (quanta). • EMR exhibits dual nature of light in that it shows both wavelike properties as well as certain characteristics of particulate matter. Copyright © 2017 Ms. Razz Chem. Class

Louis de Broglie (1924) After Einstein showed that energy displayed characteristics of matter, many wondered if matter displayed characteristics of energy. Louis de Broglie answered this question with the equation: λ= h. mv. Where, v= velocity This equation is called de Broglie’s equation. Copyright © 2017 Ms. Razz Chem. Class

Example Compare the wavelength for an electron (m= 9. 11 x 10 -31 kg) traveling at a speed of 1. 0 x 107 m/s with that for a ball (m=0. 10 kg) traveling at 35 m/s. Use: λ=h/mv h= 6. 626 x 10 -34 J·s or 6. 626 x 10 -34 kg·m 2/s (since J=kg·m 2/s 2) Electron: λ = Ball: λ= (6. 626 x 10 -34 kg·m 2/s). = 7. 27 x 10 -11 m -31 7 (9. 11 x 10 kg)(1. 0 x 10 m/s) (6. 626 x 10 -34 kg·m 2/s). (0. 10 kg)(35 m/s) = 1. 9 x 10 -34 m Copyright © 2017 Ms. Razz Chem. Class

Diffraction • Diffraction results when light is scattered from a regular array of points or lines, such as an array of atoms or ions in a crystal. • This scattered radiation produces a diffraction pattern of bright spots and dark areas on a photographic plate. • Bright spots occur because the scattered light can interfere constructively (peaks and troughs of the beams are in phase). • Dark areas occur because the scattered light can interfere destructively (peaks and troughs of the peaks are out of phase). Copyright © 2017 Ms. Razz Chem. Class

A diffraction pattern can only be explained in terms of waves. This phenomenon provides a test for the postulate that matter has wavelengths. Since a beam of electrons directed at a crystal, also produced a diffraction pattern, this verified de Broglie’s theory. Copyright © 2017 Ms. Razz Chem. Class

Light Diffraction Patterns Animation Link: Light Diffraction Copyright © 2017 Ms. Razz Chem. Class

Now it is known that matter and energy are not distinct and that energy is really a form of matter. Thus ALL matter exhibits BOTH particulate and wave properties. Copyright © 2017 Ms. Razz Chem. Class

Section 7. 3 - Atomic Spectrum of Hydrogen An important experiment was the study of the emission of light by excited hydrogen atoms. When a sample of hydrogen gas receives a high energy spark, the H 2 molecules absorb the energy and some of the H—H bonds are broken. • The resulting hydrogen atoms are excited, meaning the contain excess energy and thus release the energy through emitting light of various wavelengths to produce an emission spectrum of the hydrogen atom. Copyright © 2017 Ms. Razz Chem. Class

Continuous Spectrum • When white light is passed through a prism it contains all the wavelengths of visible light • Line spectrum – each line corresponds to a discrete wavelength: § Hydrogen emission spectrum § Only a few lines, each corresponding to a specific wavelength is seen when it is passed through a prism. Copyright © 2017 Ms. Razz Chem. Class

Animations: Continuous vs. Emission Spectra Animation Link: Continuous Spectrum of Wavelengths of Light Animation Link: Hydrogen Line Spectrum Copyright © 2017 Ms. Razz Chem. Class

The Line Spectrum: Absorption and Emission Animation Link: Light Absorption and Emission Copyright © 2017 Ms. Razz Chem. Class

Significance • Only certain energies are allowed for the electron in the hydrogen atom. • Energy of the electron in the hydrogen atom is quantized. Copyright © 2017 Ms. Razz Chem. Class

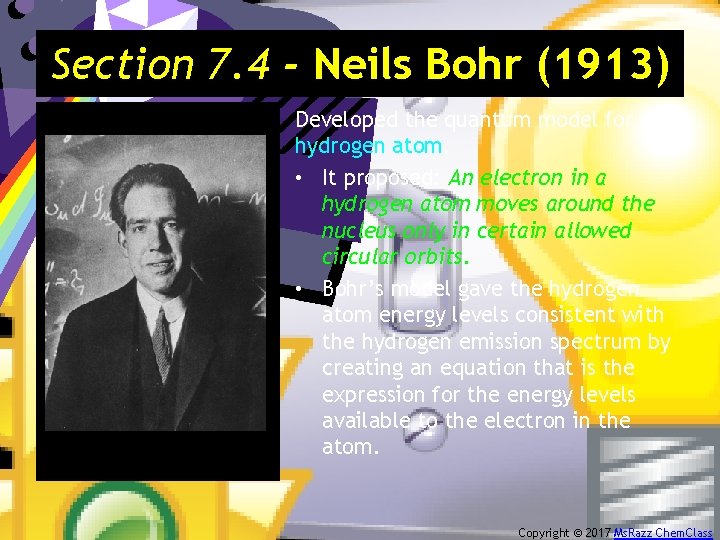
Section 7. 4 - Neils Bohr (1913) Developed the quantum model for the hydrogen atom. • It proposed: An electron in a hydrogen atom moves around the nucleus only in certain allowed circular orbits. • Bohr’s model gave the hydrogen atom energy levels consistent with the hydrogen emission spectrum by creating an equation that is the expression for the energy levels available to the electron in the atom. Copyright © 2017 Ms. Razz Chem. Class

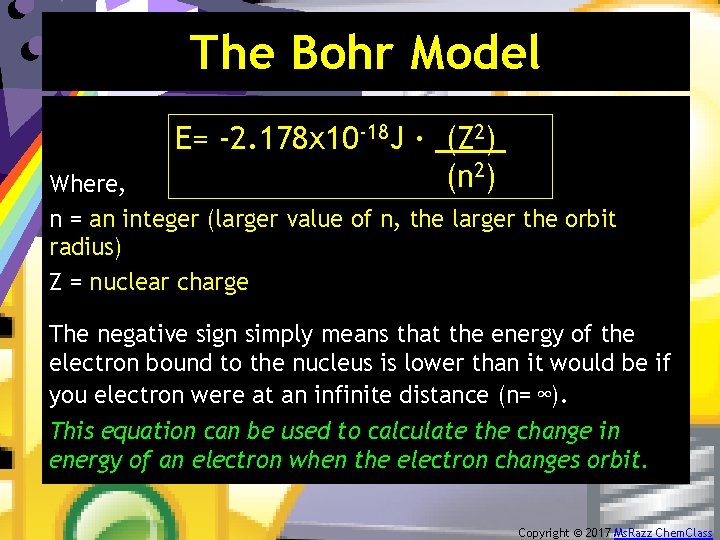
The Bohr Model E= -2. 178 x 10 -18 J · (Z 2). (n 2) Where, n = an integer (larger value of n, the larger the orbit radius) Z = nuclear charge The negative sign simply means that the energy of the electron bound to the nucleus is lower than it would be if you electron were at an infinite distance (n= ∞). This equation can be used to calculate the change in energy of an electron when the electron changes orbit. Copyright © 2017 Ms. Razz Chem. Class

The wavelength of the emitted photon can then be calculated from the equation: ∆E= hc λ The ground state is the lowest possible energy state. ∆E= energy of final state - energy of initial state Copyright © 2017 Ms. Razz Chem. Class

Electronic Transitions in Animation Link: the Bohr Electron Transitions in Model for the Hydrogen Atom Copyright © 2017 Ms. Razz Chem. Class

Example Calculate the energy required to excite the hydrogen electron from level n=1 to level n=2. Also calculate the wavelength of light that must be absorbed by a hydrogen atom in its ground state to reach this excited state. E 1= -2. 178 x 10 -18 J · (12). = -2. 178 x 10 -18 J (12) E 2= -2. 178 x 10 -18 J · (12). = -5. 445 x 10 -19 J (22) So, ∆E= (-5. 445 x 10 -19) - (-2. 178 x 10 -18 J) ∆E= +1. 633 x 10 -18 J The positive sign indicates that system has gained energy. Thus the wavelength must be absorbed. Copyright © 2017 Ms. Razz Chem. Class

Example (cont. ) Calculate the energy required to excite the hydrogen electron from level n=1 to level n=2. Also calculate the wavelength of light that must be absorbed by a hydrogen atom in its ground state to reach this excited state. λ=hc/∆E λ= (6. 626 x 10 -34 J·s) (2. 9979 x 108 m/s) 1. 633 x 10 -18 J = 1. 216 x 10 -7 m Copyright © 2017 Ms. Razz Chem. Class

Bohr Model of the Atom Two important points about the Bohr model: • It correctly fits the quantized energy levels of the hydrogen atom and postulates only certain allowed circular orbits for the electron. • As electron becomes more tightly bound, or as n becomes smaller and smaller, its energy becomes more negative. – Energy is released when an electron is brought closer to the nucleus. Copyright © 2017 Ms. Razz Chem. Class

But… • Bohr’s model is incorrect. This model only works for hydrogen. • Electrons do not move around the nucleus in circular orbits. Copyright © 2017 Ms. Razz Chem. Class

An important derivation of the original Bohr equation can make calculating the change in energy easier. Copyright © 2017 Ms. Razz Chem. Class

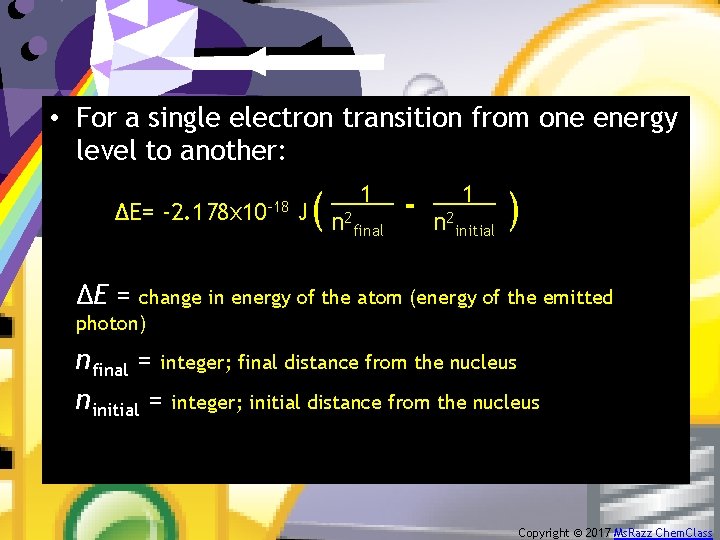
• For a single electron transition from one energy level to another: ∆E= -2. 178 x 10 -18 ( 1. J n 2 final - 1. n 2 initial ) ΔE = change in energy of the atom (energy of the emitted photon) nfinal = integer; final distance from the nucleus ninitial = integer; initial distance from the nucleus Copyright © 2017 Ms. Razz Chem. Class

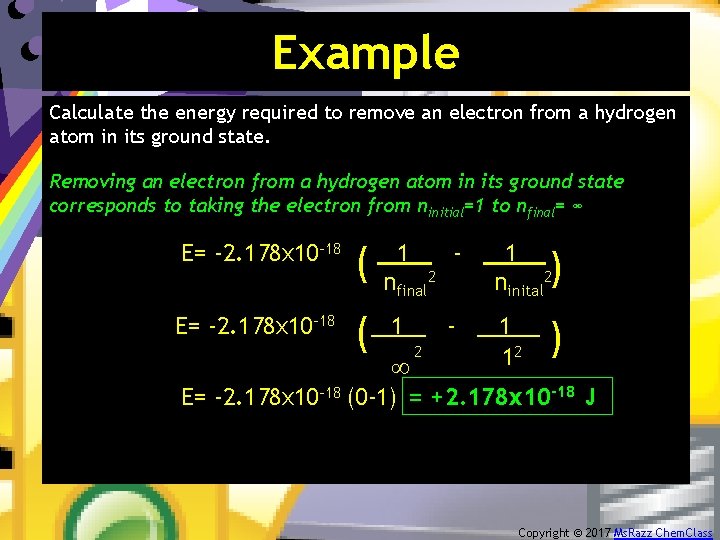
Example Calculate the energy required to remove an electron from a hydrogen atom in its ground state. Removing an electron from a hydrogen atom in its ground state corresponds to taking the electron from ninitial=1 to nfinal= ∞ ( 1 - 1. ninital 2 - 1. 12 nfinal 2 1 ∞ 2 ( E= -2. 178 x 10 -18 (0 -1) = +2. 178 x 10 -18 J Copyright © 2017 Ms. Razz Chem. Class

Bohr’s model was very promising at first, but when it was applied to other atoms other than hydrogen, it did not work at all. This showed that there was fundamental error in the formula. Though no current theories of atomic structure are derived from it, it did show that quantization of energy could be explained through easy assumptions. Copyright © 2017 Ms. Razz Chem. Class

Section 7. 5 – Quantum Mechanical Model of the Atom When it became apparent that the Bohr model was flawed, a new approach was developed called quantum mechanics. This approach focuses on the wave properties of the electron. Copyright © 2017 Ms. Razz Chem. Class

Standing Waves • Scientists realized that the electrons bound to the nucleus seemed similar to a standing wave, a wave that is stationary. • Example of a standing wave is the plucking of any musical instrument like a guitar. Copyright © 2017 Ms. Razz Chem. Class

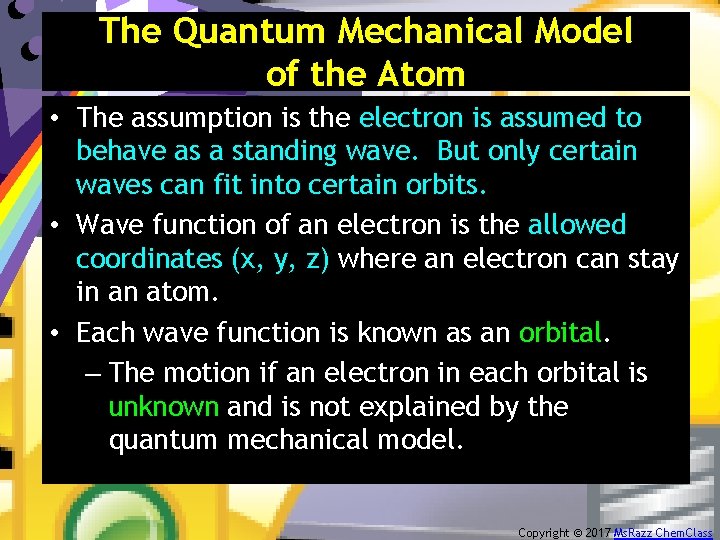
The Quantum Mechanical Model of the Atom • The assumption is the electron is assumed to behave as a standing wave. But only certain waves can fit into certain orbits. • Wave function of an electron is the allowed coordinates (x, y, z) where an electron can stay in an atom. • Each wave function is known as an orbital. – The motion if an electron in each orbital is unknown and is not explained by the quantum mechanical model. Copyright © 2017 Ms. Razz Chem. Class

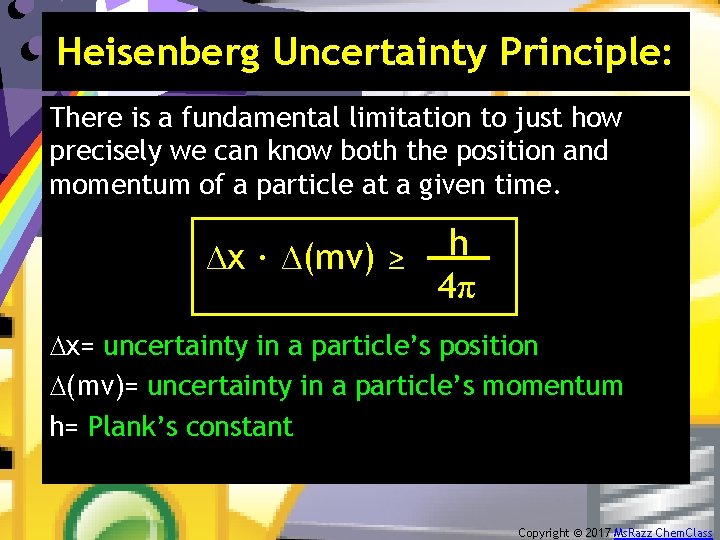
Heisenberg Uncertainty Principle: There is a fundamental limitation to just how precisely we can know both the position and momentum of a particle at a given time. h. ∆x ∙ ∆(mv) ≥ 4π ∆x= uncertainty in a particle’s position ∆(mv)= uncertainty in a particle’s momentum h= Plank’s constant Copyright © 2017 Ms. Razz Chem. Class

Uncertainty Principle • This states that you cannot predict exactly where an electron is going to be; but, can know the probability of it being within a certain location (the orbitals are the areas of probability for locating electrons) • The square of the wave function is called probability distribution which represents the probability of finding the electron in a particular place around the atom. Animation Link: Probability Electron Cloud Copyright © 2017 Ms. Razz Chem. Class

• As you move away from the nucleus, the probability of finding the electron at a given position decreases. • We can use theories to refine the hydrogen 1 s orbital, the size of it is the radius of the sphere that encloses 90% of the total electron probability. Copyright © 2017 Ms. Razz Chem. Class

Physical Meaning of a Wave Function The square of the function indicates the probability of finding an electron near a particular point in space. • Probability distribution – intensity of color is used to indicate the probability value near a given point in space. • Picture an orbital as a three-dimensional electron density map. Copyright © 2017 Ms. Razz Chem. Class

Section 7. 6 – Quantum Numbers • Principal quantum number (n) – size and energy of the orbital. Has integral values: 1, 2, 3, 4, … – As n increases the orbital becomes larger and the energy increases because it is farther away from the nucleus. Copyright © 2017 Ms. Razz Chem. Class

Quantum Numbers • Angular momentum quantum number (l) – shape of atomic orbitals (sometimes called a subshell). – Has integral values from 0 to n-1 for each value of n. – The value of l for a particular orbital is commonly assigned a letter: The Angular Momentum Quantum Numbers and Corresponding Letters Used to Designate Atomic Orbitals Value of l 0 1 2 3 4 Letter Used s p d f g Copyright © 2017 Ms. Razz Chem. Class

Quantum Numbers • Magnetic quantum number (ml) – orientation of the orbital in space relative to the other orbitals in the atom. – Has integral values between l and -l, including zero. Copyright © 2017 Ms. Razz Chem. Class

Quantum Numbers • Magnetic spin quantum number (ms) – Represents the two electrons that can go into a particular orbital. – Has two possible values: +1/2 and -1 / 2. Copyright © 2017 Ms. Razz Chem. Class

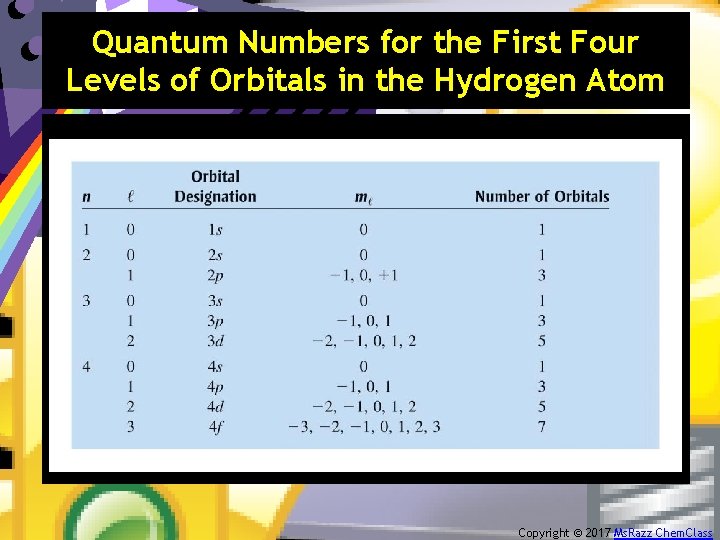
Quantum Numbers for the First Four Levels of Orbitals in the Hydrogen Atom Copyright © 2017 Ms. Razz Chem. Class

Example For principal quantum level n = 3, determine the number of allowed subshells (different values of l), and give the designation of each. # of allowed subshells = 3 l = 0, 3 s l = 1, 3 p l = 2, 3 d Copyright © 2017 Ms. Razz Chem. Class

Example What are the sets of quantum numbers for nitrogen’s 7 electrons in their ground state? In the first energy level: (1, In the second energy level: (2, (2, 0, 0, +1/2) (1, 0, 0, -1/2) 0, 0, +1/2) (2, 0, 0, -1/2) 1, -1, +1/2) (2, 1, 0, +1/2) 1, 1, +1/2) Notice how the three electrons in the separate p orbitals have identical spins. Copyright © 2017 Ms. Razz Chem. Class

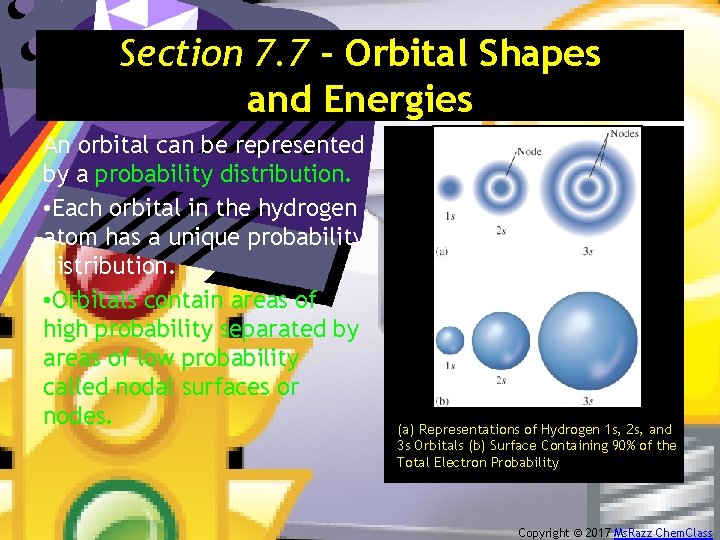
Section 7. 7 - Orbital Shapes and Energies An orbital can be represented by a probability distribution. • Each orbital in the hydrogen atom has a unique probability distribution. • Orbitals contain areas of high probability separated by areas of low probability called nodal surfaces or nodes. (a) Representations of Hydrogen 1 s, 2 s, and 3 s Orbitals (b) Surface Containing 90% of the Total Electron Probability Copyright © 2017 Ms. Razz Chem. Class

S orbitals • s orbitals first occur in n=1. – They have an overall spherical shape, which becomes larger as the value of n increases. – Animation Link: 1 s orbital Copyright © 2017 Ms. Razz Chem. Class

The Boundary Surface Representations of All Three 2 p Orbitals • p orbitals first occur in n=2. – p orbitals have two lobes separated by a node as the nucleus. – There are three p orbitals in each sublevel. They are labeled according to the axis of the xyz coordinate system along the lobes lie. (px, py, pz) – Animation Links: 2 px orbital, 2 py orbital, 2 pz orbital Copyright © 2017 Ms. Razz Chem. Class

The Boundary Surfaces of All of the 3 d Orbitals • d orbitals first occur in n=3. –There are five d orbitals in each sublevel. –Four of the d orbitals (xz, yz, xy, x 2 -y 2) have four lobes centered in the plane indicated in the orbitals label. –The fifth orbital (z 2) has a unique shape with two lobes long the z axis and a belt centered by the xy plane. –Animation Links: 3 xz, 3 yz, 3 dxy, 3 dx 2 -y 2, 3 z 2 Copyright © 2017 Ms. Razz Chem. Class

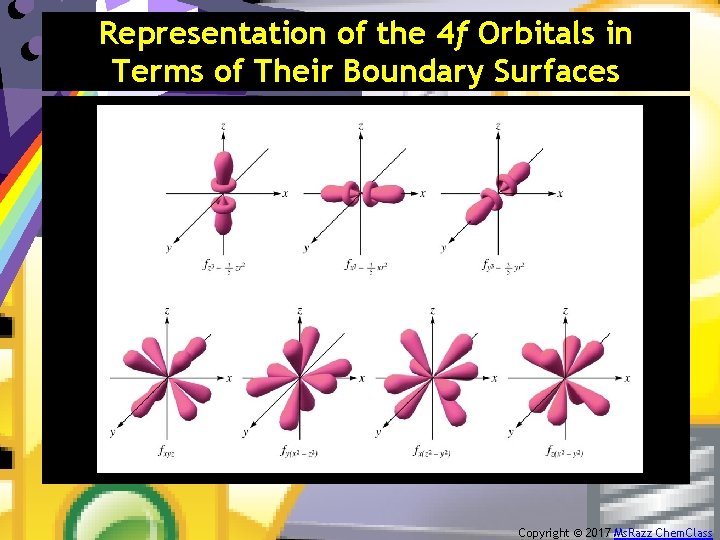
Representation of the 4 f Orbitals in Terms of Their Boundary Surfaces • f orbitals first occur in n=4. – There are seven f orbitals in each sublevel. – The shapes of f orbitals are very complex. Copyright © 2017 Ms. Razz Chem. Class

Representation of the 4 f Orbitals in Terms of Their Boundary Surfaces Copyright © 2017 Ms. Razz Chem. Class

Section 7. 8 - Electron Spin and the Pauli Principle • A fourth quantum number was needed to account of the emission spectra of atoms. • Electron spin quantum number (ms) – can be +½ or -½. Copyright © 2017 Ms. Razz Chem. Class

Electron Spin Austrian physicist Wolfgang Pauli developed the Pauli Exclusion Principle: • In a given atom no two electrons can have the same set of four quantum numbers. • An orbital can hold only two electrons, and they must have opposite spins. Copyright © 2017 Ms. Razz Chem. Class

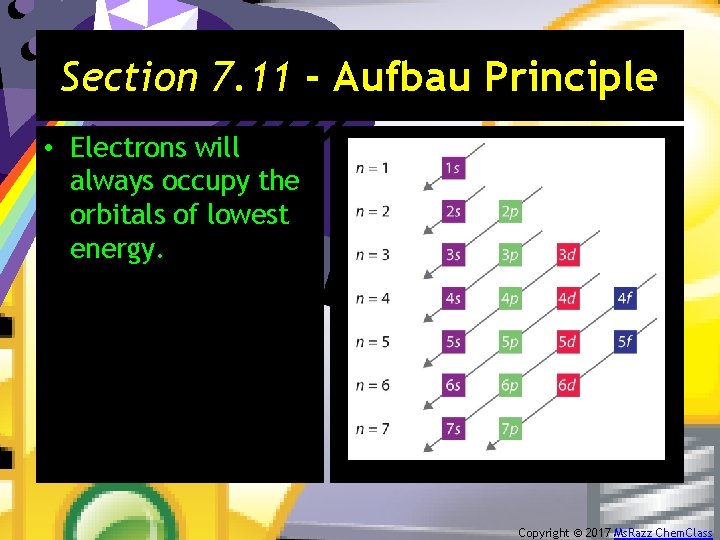
Section 7. 9 - Polyelectronic Atoms • Atoms with more than one electron. • Electron correlation problem: – Since the electron pathways are unknown, the electron repulsions cannot be calculated exactly. • When electrons are placed in a particular quantum level, they “prefer” the orbitals in the order s, p, d, and then f. Copyright © 2017 Ms. Razz Chem. Class

The QM model gives a description of the hydrogen atom that agrees very well with experimental data. However, the model would not be a very good one if it did not account for the properties of all the other atoms as well. These other atoms are called polyelectronic atoms. Copyright © 2017 Ms. Razz Chem. Class

Quantum Mechanical Model for Polyelectronic Atoms To see how the QM model applies to polyelectronic atoms, let’s consider helium. Three energy contributions must be considered in the description of the helium atom: • The kinetic energy of the electrons as they move around the nucleus. • The potential energy of the attraction between the nucleus and the electron. • The potential energy of repulsion between two electrons. Copyright © 2017 Ms. Razz Chem. Class

Problem… The helium atom can be described in terms of the quantum mechanical model. But the equation that results cannot be solved exactly due to the repulsions between electrons. • Because the electron pathways are unknown, the electron repulsions cannot be calculated exactly – this is called the electron correlation problem. – Occurs with all polyelectronic atoms. Copyright © 2017 Ms. Razz Chem. Class

• To account for this problem, we have to make approximations. We have to treat each electron as if it were moving in a field of charge that is the net result of the nuclear attraction and the average repulsions of all the other electrons. Copyright © 2017 Ms. Razz Chem. Class

Hydrogen vs. Polyelectronic Atoms • One major difference between the hydrogen atom and a polyelectronic atom is that in a hydrogen atom all the orbitals in a given principal quantum level have the same energy (they are said to be degenerate). • This is not the case in polyelectronic atoms. Copyright © 2017 Ms. Razz Chem. Class

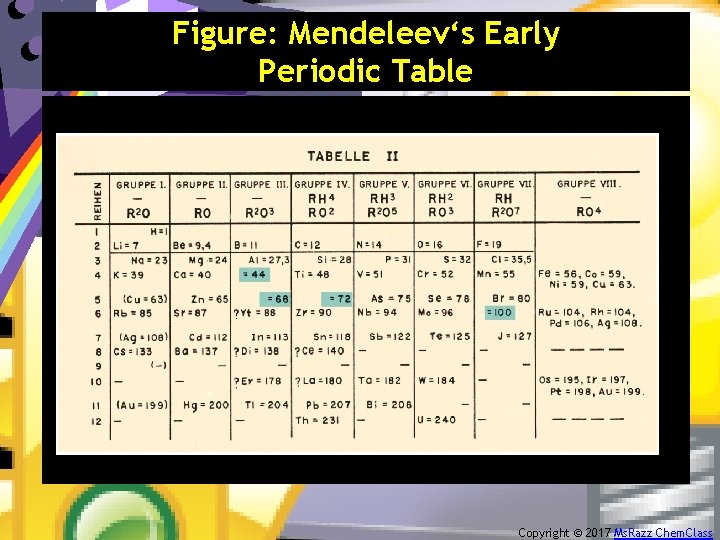
Section 7. 10 – The History of the Periodic Table The present form of the Periodic Table was conceived independently by Julius Meyer (1830 -1895) and Dmitri Mendeleev (1834 -1907). • Mendeleev is given the most credit because it was he who emphasized how useful the table could be in predicting the existence and properties of still unknown elements. Copyright © 2017 Ms. Razz Chem. Class

• In 1872 Mendeleev predicted correctly that gallium, scandium, and germanium existed. • Furthermore, he correctly predicted the atomic masses of several elements like Indium. • Mendeleev ordered the elements according to increasing atomic mass and placed elements with similar properties together in the same column. • Mosely later rearranged the elements according to increasing atomic number. Copyright © 2017 Ms. Razz Chem. Class

Figure: Mendeleev‘s Early Periodic Table Copyright © 2017 Ms. Razz Chem. Class

Section 7. 11 - Aufbau Principle • Electrons will always occupy the orbitals of lowest energy. Copyright © 2017 Ms. Razz Chem. Class

Example What is the electron configuration of Manganese? Mn (25 e-): 1 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 2 Copyright © 2017 Ms. Razz Chem. Class

Hund’s Rule • The lowest energy configuration for an atom is the one having the maximum number of unpaired electrons allowed by the Pauli principle in a particular set of degenerate (same energy) orbitals. Copyright © 2017 Ms. Razz Chem. Class

Orbital Diagram • A notation that shows how many electrons an atom has in each of its occupied electron orbitals. Oxygen: 1 s 22 p 4 Oxygen: 1 s 2 s 2 p Copyright © 2017 Ms. Razz Chem. Class

Valence Electrons • The electrons in the outermost principal quantum level of an atom. 1 s 22 p 6 (valence electrons = 8) • The elements in the same group on the periodic table have the same valence electron configuration. Copyright © 2017 Ms. Razz Chem. Class

Exceptions to Filling There are numerous exceptional electron configurations. Some common exceptions are the result of the stability of half-filled and filled d and f sublevels. Elements that have expected configurations ending in d 4, d 9, f 6, or f 13 will commonly “borrow” an electron from the outermost s sublevel to make half-filled or filled d or f sublevels. Copyright © 2017 Ms. Razz Chem. Class

Exceptions to Filling Example: Cu has an expected electron configuration of 1 s 2 2 p 6 3 s 2 3 p 6 3 d 9 4 s 2 But its actual configuration is: 1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 1 Copyright © 2017 Ms. Razz Chem. Class

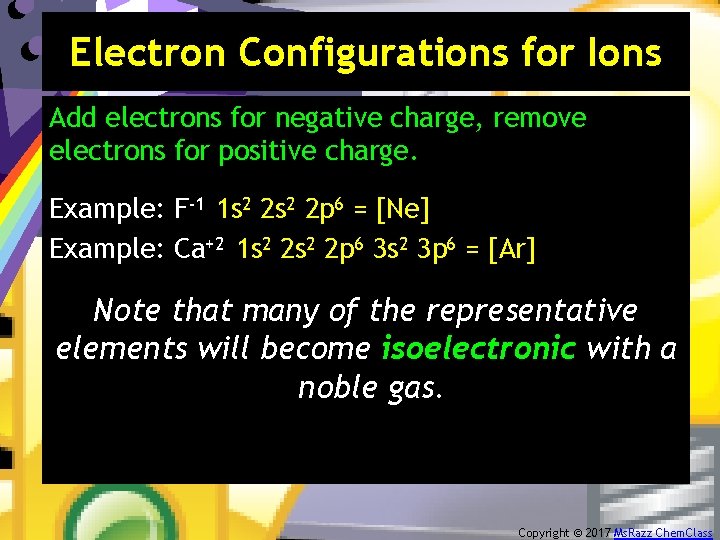
Electron Configurations for Ions Add electrons for negative charge, remove electrons for positive charge. Example: F-1 1 s 2 2 p 6 = [Ne] Example: Ca+2 1 s 2 2 p 6 3 s 2 3 p 6 = [Ar] Note that many of the representative elements will become isoelectronic with a noble gas. Copyright © 2017 Ms. Razz Chem. Class

Ions: Transition Metals To make ions of transition metals, be sure to remove the valence electrons before removing any core electrons. Fe Fe+2 Fe+3 1 s 2 2 p 6 3 s 2 3 p 6 3 d 6 4 s 2 1 s 2 2 p 6 3 s 2 3 p 6 3 d 6 1 s 2 2 p 6 3 s 2 3 p 6 3 d 5 Copyright © 2017 Ms. Razz Chem. Class

Section 7. 12 - Periodic Trends • Ionization Energy • Electron Affinity • Atomic Radius Copyright © 2017 Ms. Razz Chem. Class

Ionization Energy • Energy required to remove an electron from a gaseous atom or ion. X(g) → X+(g) + e– Mg → Mg+ + e– I 1 = 735 k. J/mol (1 st IE) Mg+ → Mg 2+ + e– I 2 = 1445 k. J/mol (2 nd IE) Mg 2+ → Mg 3+ + e– I 3 = 7730 k. J/mol *(3 rd IE) *Core electrons are bound much more tightly than valence electrons. Copyright © 2017 Ms. Razz Chem. Class

Ionization Energy (cont. ) Ionization energy always increases with each successive removal because the electrons are being removed from an ion which is increasingly positive in charge. Copyright © 2017 Ms. Razz Chem. Class

Group Trend for Ionization Energy • In general, as we go down a group from top to bottom, the first ionization energy decreases. • Why? The electrons being removed are farther from the nucleus. Copyright © 2017 Ms. Razz Chem. Class

Period Trend for Ionization Energy • In general, as we go across a period from left to right, the first ionization energy increases. • Why? Electrons in the same principal quantum level are generally more strongly bound from left to right on the Periodic Table due to increased nuclear charge. Copyright © 2017 Ms. Razz Chem. Class

Exceptions to the Period Trend for Ionization Energy There are some exceptions in the period trend though. • There is a decrease in ionization energy: – In group 13 (due to increased shielding provided by the filled s orbital). – In group 16 (due to the added electron repulsions when the electrons begin pairing in the p sublevel). Copyright © 2017 Ms. Razz Chem. Class

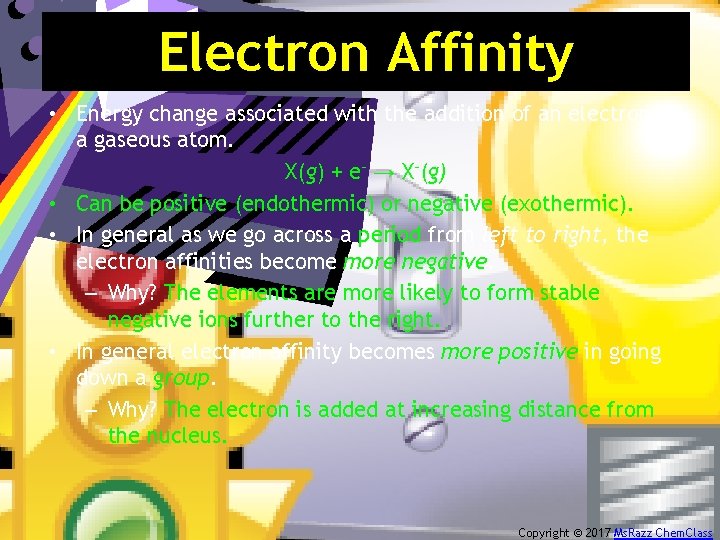
Electron Affinity • Energy change associated with the addition of an electron to a gaseous atom. X(g) + e– → X–(g) • Can be positive (endothermic) or negative (exothermic). • In general as we go across a period from left to right, the electron affinities become more negative. – Why? The elements are more likely to form stable negative ions further to the right. • In general electron affinity becomes more positive in going down a group. – Why? The electron is added at increasing distance from the nucleus. Copyright © 2017 Ms. Razz Chem. Class

Electron Affinity However, there are multiple exceptions to this rule. • For example, Beryllium and Nitrogen do not form stable -1 ions due to the added repulsions between the electrons in the doubly occupied orbital. Copyright © 2017 Ms. Razz Chem. Class

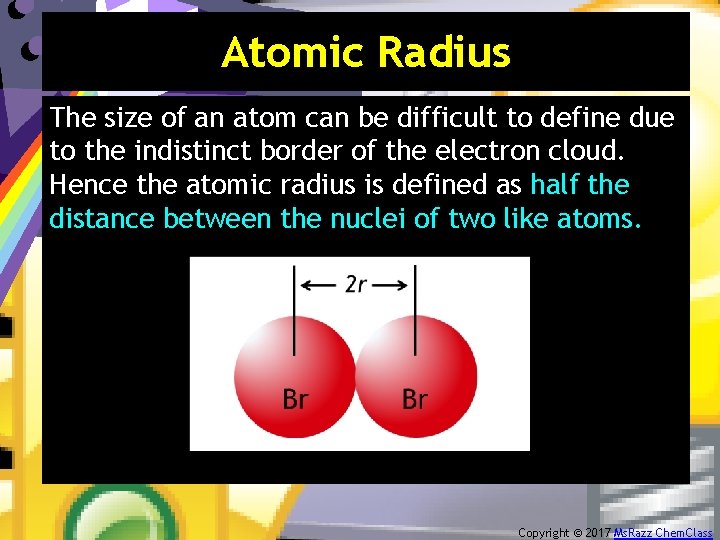
Atomic Radius The size of an atom can be difficult to define due to the indistinct border of the electron cloud. Hence the atomic radius is defined as half the distance between the nuclei of two like atoms. Copyright © 2017 Ms. Razz Chem. Class

Atomic Radius Group Trend • In general atomic radius increases in going down a group due to a greater number of occupied energy levels. • Note that most of an atom’s size is due to the space occupied by the electrons. Therefore an atom that has more occupied energy levels will always be larger. Examples: Li 1 s 2 2 s 1 Na 1 s 2 2 p 6 3 s 1 K 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 Rb 1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 5 s 1 Copyright © 2017 Ms. Razz Chem. Class

Atomic Radius Period Trend • In general as we go across a period from left to right, the atomic radius decreases due to the increased nuclear charge. – Why? Effective nuclear charge increases, therefore the valence electrons are drawn closer to the nucleus, decreasing the size of the atom. Copyright © 2017 Ms. Razz Chem. Class

Ionic Size Cations are always smaller than the neutral atom from which they are made because they have lost electrons. Sometimes this will include the loss of an occupied energy level. The more electrons lost, the smaller the ion becomes due to the increased proton: electron ratio. Example: Mg > Mg 1+ > Mg 2+ Copyright © 2017 Ms. Razz Chem. Class

Ionic Size Anions are always larger than the neutral atom from which they are made because they have gained electrons and the proton: electron decreases. The more electrons gained, the larger the ion will become. Example: O < O 1 - < O 2 - Copyright © 2017 Ms. Razz Chem. Class

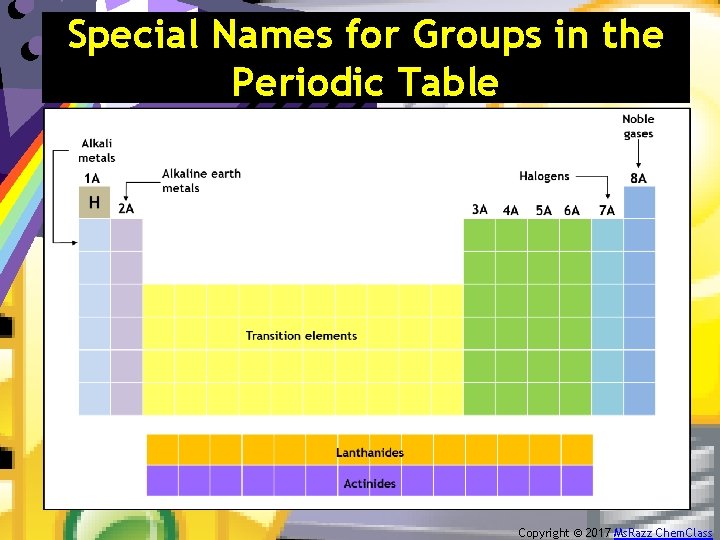
The Periodic Table – Summary 1. It is the number and type of valence electrons that primarily determine an atom’s chemistry. 2. Electron configurations can be determined from the organization of the periodic table. 3. Certain groups in the periodic table have special names. Copyright © 2017 Ms. Razz Chem. Class

Special Names for Groups in the Periodic Table Copyright © 2017 Ms. Razz Chem. Class

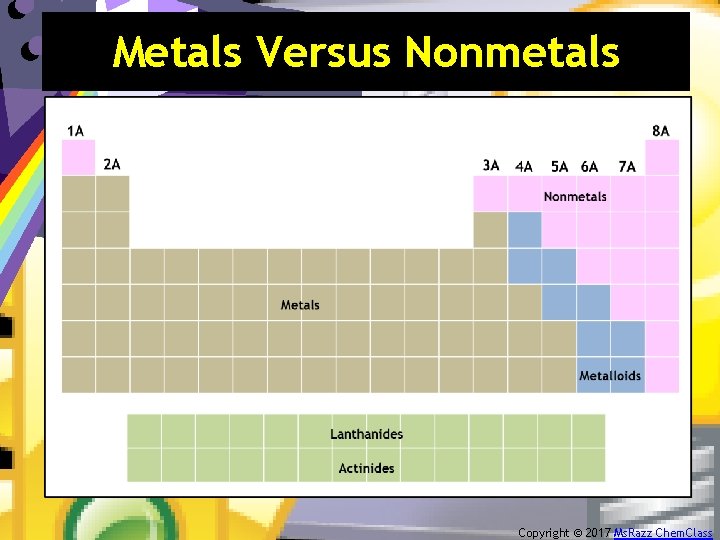
The Periodic Table – Final Thoughts 4. Basic division of the elements is into metals and nonmetals. Copyright © 2017 Ms. Razz Chem. Class

Metals Versus Nonmetals Copyright © 2017 Ms. Razz Chem. Class

Section 7. 13 - The Alkali Metals • • Li, Na, K, Rb, Cs, and Fr Most chemically reactive of the metals React with nonmetals to form ionic solids Ionization energy decreases down the group Atomic radius increases down the group Density increases (Typical of all groups) Melting and boiling points smoothly decrease down the group which is not typical. Group 17 MP and BP will increase down a group. Other groups exhibit more complicated behavior. Copyright © 2017 Ms. Razz Chem. Class

The Alkali Metals (cont. ) • They react with halogens to form salts. 2 Na + Cl 2 2 Na. Cl (act as reducing agents) • They also react violently with water. • The have a large hydration energy (the energy change that occurs when water molecules attach to an ion). Copyright © 2017 Ms. Razz Chem. Class