Chemistry Review Atomic Theory and Atomic Structure Who























- Slides: 32

Chemistry Review Atomic Theory and Atomic Structure

Who am I?

Responsible for naming the atom 1. 2. 3. 4. Dalton Bohr Rutherford Democritus

Who am I?

I proposed the atomic theory. 1. 2. 3. 4. 5. Bohr Rutherford Chadwick Dalton Democritus

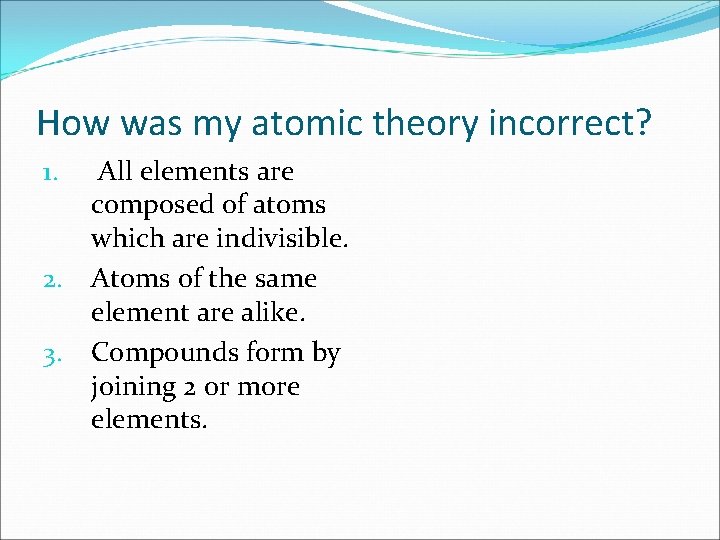
How was my atomic theory incorrect? All elements are composed of atoms which are indivisible. 2. Atoms of the same element are alike. 3. Compounds form by joining 2 or more elements. 1.

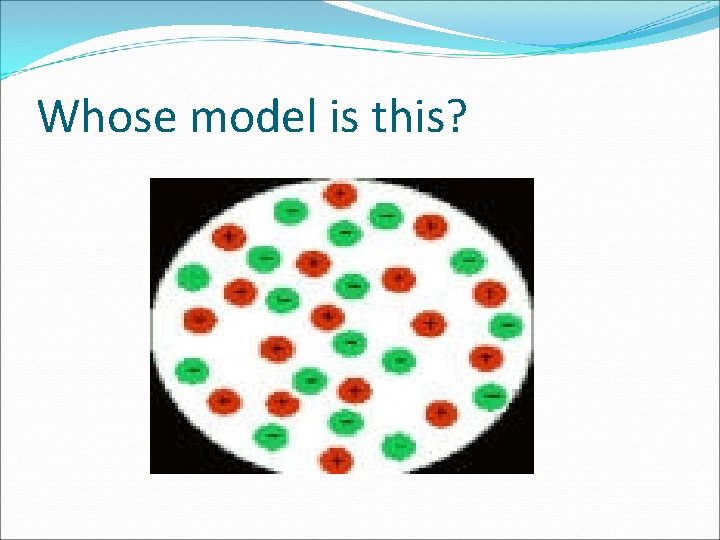
Whose model is this?

The previous model showed positive and negative charges mixed like “Plum Pudding” 1. 2. 3. 4. Bohr Dalton Thomson Rutherford

Who am I and here is my model

Discovered the nucleus had a positive charge with electrons outside of it. 1. 2. 3. 4. Bohr Dalton Rutherford Thomson

Rutherford Gold Foil Experiment The reflected alpha particles bounced off of the positively charged nucleus – which proved the existence of the nucleus. True or False? True – alpha particles are positive The reflected alpha particles bounced off of the atoms in the gold foil – which proved the existence of atoms. True or False? False

Rutherford Gold Foil Experiment Most of the alpha particles went straight through the gold foil undeflected – which proved that the positive charge was spread out evenly through the atom. True or False. Positive would reflect positive.

Who am I ?

My contribution to the atomic theory development was Nothing, I was a loser. Discovered protons. Placed electrons in specific energy levels. 4. Discovered neutrons. 1. 2. 3.

Who am I ?

Who am I and what did I do? Rutherford and discovered protons and nucleus. 2. Thompson and discovered electrons. 3. Chadwick, and discovered neutrons. 1.

Why were the neutrons so difficult to detect? 1. 2. 3. Because they are so massive. Because they have no charge. Because they have numerous charges.

Subatomic Particles Particle Symbol Charge Electron e- - Proton p+ + Neutron n neutral Relative Mass 0 1 1

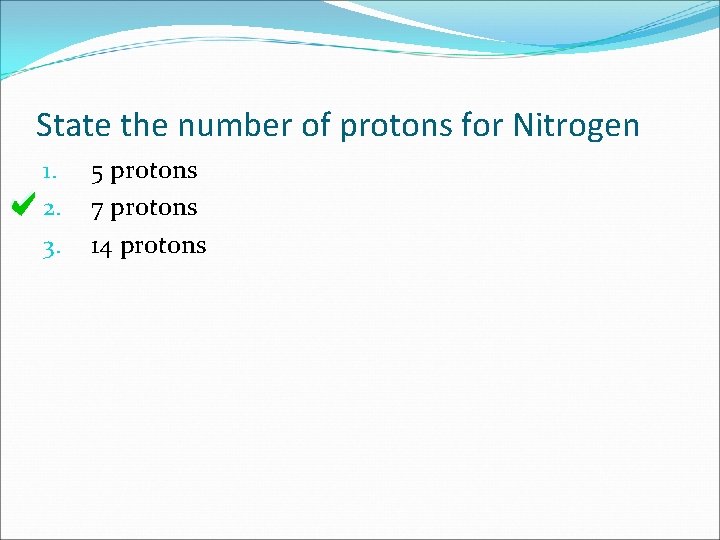
State the number of protons for Nitrogen 1. 2. 3. 5 protons 7 protons 14 protons

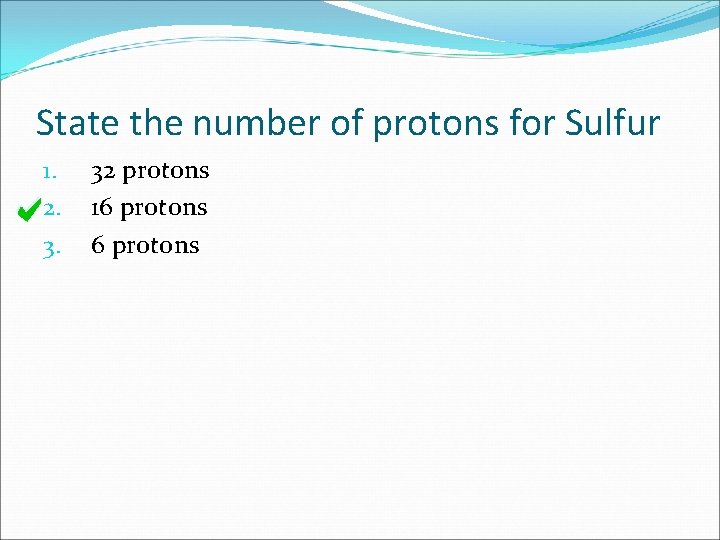
State the number of protons for Sulfur 1. 2. 3. 32 protons 16 protons

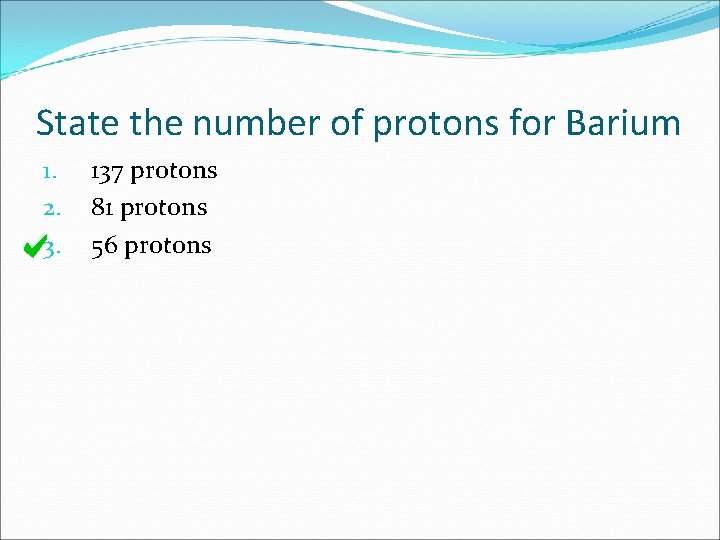
State the number of protons for Barium 1. 2. 3. 137 protons 81 protons 56 protons

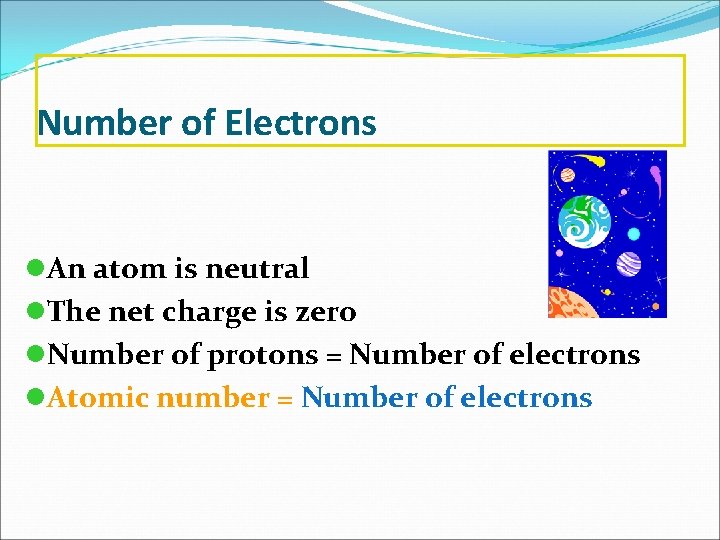
Number of Electrons l. An atom is neutral l. The net charge is zero l. Number of protons = Number of electrons l. Atomic number = Number of electrons

Ions – charged atoms If an ion gains an electron it becomes _______ charged and is called an _______. Answer: negatively anion

Ions: charged atoms If an ion loses an electron , it becomes ______ charged, and is called a _______. Answer: positively, cation

An atom of zinc has a mass number of 65. Number of protons in zinc are 1. 2. 3. 4. 30 35 65 125

The number of neutrons in zinc are 1. 2. 3. 4. 30 35 65 125

What is the mass number of a zinc isotope with 37 neutrons? 1. 2. 3. 4. 37 65 67 125

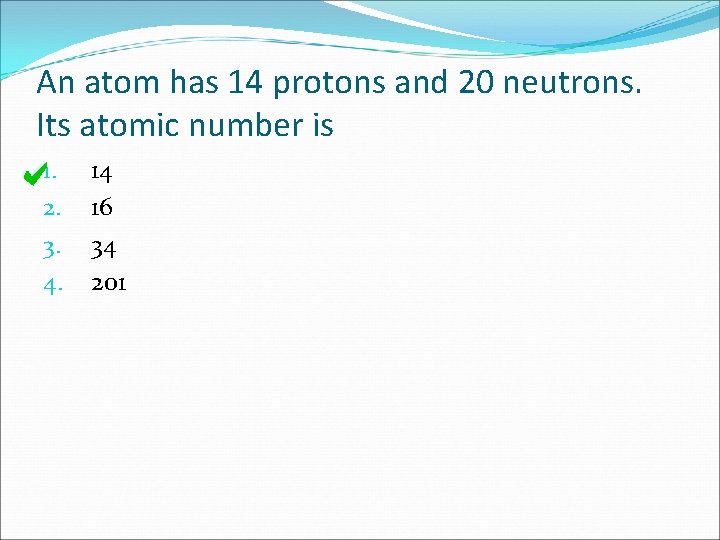
An atom has 14 protons and 20 neutrons. Its atomic number is 1. 2. 3. 4. 14 16 34 201

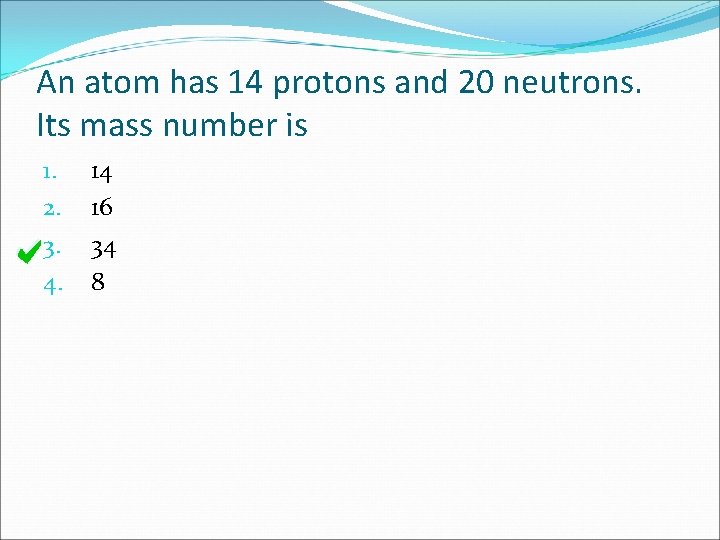
An atom has 14 protons and 20 neutrons. Its mass number is 1. 2. 3. 4. 14 16 34 8

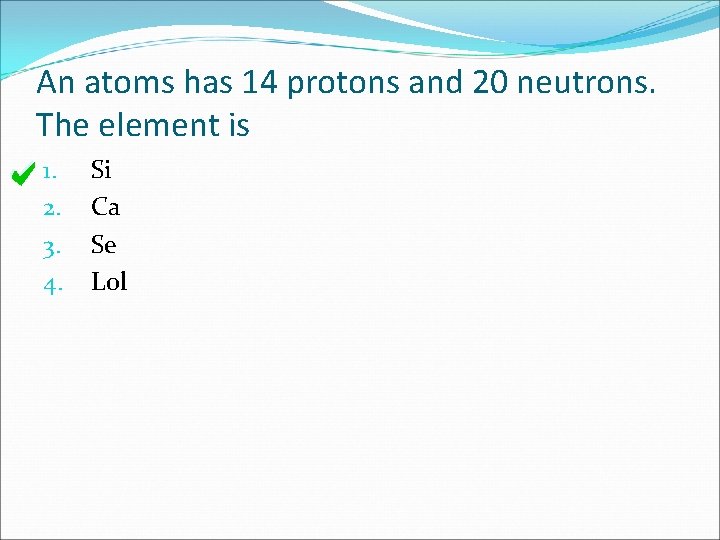
An atoms has 14 protons and 20 neutrons. The element is 1. 2. 3. 4. Si Ca Se Lol

If an element has 12 protons and 12 neutrons, how many electrons are found in the nucleus? A. 12 B. 24 C. Only the ones with lots of energy. D. No electrons are found in nucleus.

Ions 37 Cl -1 17 How many protons? How many neutrons? How many electrons? Anion or Cation?
 Atomic structure and properties ap chemistry
Atomic structure and properties ap chemistry Ap chemistry chapter 7
Ap chemistry chapter 7 Oxygen periodic trends
Oxygen periodic trends First ionization energy of calcium
First ionization energy of calcium What is calcium ionization energy
What is calcium ionization energy Relative atomic mass of beryllium
Relative atomic mass of beryllium Mass of oxygen
Mass of oxygen Difference between atomic number and atomic mass
Difference between atomic number and atomic mass Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Chemistry grade 10 review
Chemistry grade 10 review Periodic trneds
Periodic trneds Atomic radius in periodic table
Atomic radius in periodic table Atomic number vs atomic radius
Atomic number vs atomic radius Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Atomic model and theory timeline
Atomic model and theory timeline Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry What is the chemical formula for tetranitrogen heptoxide?
What is the chemical formula for tetranitrogen heptoxide? Chapter 14 review acids and bases
Chapter 14 review acids and bases Modern chemistry chapter 13 review answers
Modern chemistry chapter 13 review answers Chapter 12 solutions chemistry
Chapter 12 solutions chemistry Chemistry sol review packet
Chemistry sol review packet Chemistry unit review answer key
Chemistry unit review answer key Kuhinjska sol formula
Kuhinjska sol formula What functional group is ch3
What functional group is ch3 Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Ap chemistry equilibrium review
Ap chemistry equilibrium review Ap chemistry big idea 2 review answers
Ap chemistry big idea 2 review answers Chemistry sol review
Chemistry sol review Chemistry sol review
Chemistry sol review Chemistry sol review
Chemistry sol review Chemistry midterm review
Chemistry midterm review