Atomic Theory Chemistry Early Atomic Theories first theory







- Slides: 23

Atomic Theory Chemistry

Early Atomic Theories ¡- first theory of elements was by Greek philosopher Plato (440 BC) ¡ his student Aristotle carried out his work (384 -322 BC) ¡ emphasized the five elements that all matter is made of: ¡ 1 ¡ 2 ¡ 3 ¡ 4 ¡ 5

Indivisible particles ¡the less popular theory was by the philosopher Democritus(470 -370 BC) ¡ all matter was divisible up to a certain point ¡ you eventually get down to a particle that is indivisible ¡ the Greek word translates to “not cuttable” or atomos ¡Aristotle’s idea was followed for several centuries

John Dalton ¡first atomic theory 1803 -1810, with the criteria: ¡ Elements are composed small, indivisible particles called atoms ¡ Atoms of the same element have the same size and weight ¡ Atoms of different elements have different size and weight ¡ Atoms cannot be created nor destroyed ¡ Compounds are formed by the addition of two or more different elements in simple whole number ratios ¡ In chemical reactions, atoms are combined, separated, or generally rearranged

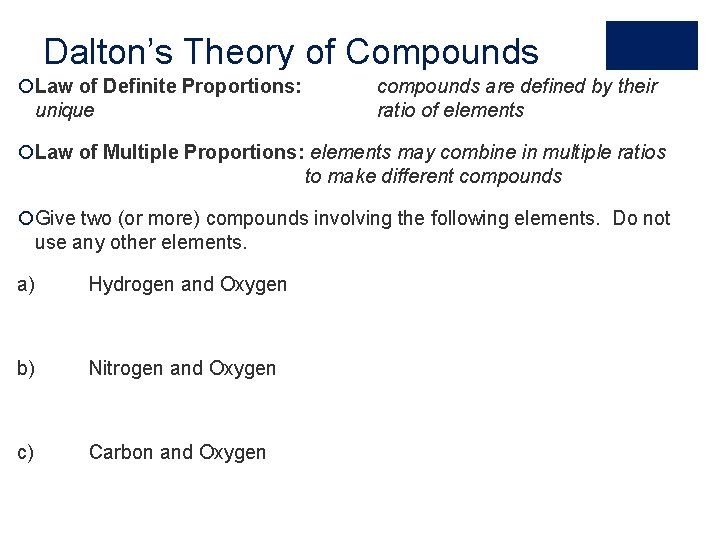
Dalton’s Theory of Compounds ¡Law of Definite Proportions: unique compounds are defined by their ratio of elements ¡Law of Multiple Proportions: elements may combine in multiple ratios to make different compounds ¡Give two (or more) compounds involving the following elements. Do not use any other elements. a) Hydrogen and Oxygen b) Nitrogen and Oxygen c) Carbon and Oxygen

Antoine Lavoisier (late 1700 s) ¡Early experiments in quantitative chemistry ¡ Oxygen theory of combustion (opposes phlogiston theory) ¡Age 28 marries Marie-Anne Paulze (13 years old) ¡ Favor to his boss ¡ Expert on language, translates other chemist works ¡ Lab tech, writes all reports, technical drawings ¡Executed during French revolution ¡ Wife rescued his works


Joseph Priestly (1700 s) • Highly religious Christian scientist, known as a founder of the Unitarians • Experimented and observed several different gases or “ airs“, notably: • Isolated and discovered pure, elemental oxygen Found its connection with respiration, and its function in blood • Unfortunately connected many ideas to phlogiston theory

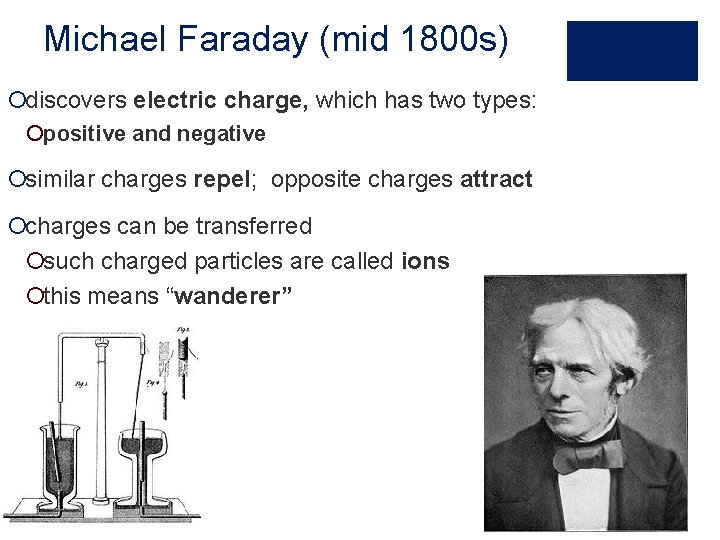
Michael Faraday (mid 1800 s) ¡discovers electric charge, which has two types: ¡positive and negative ¡similar charges repel; opposite charges attract ¡charges can be transferred ¡such charged particles are called ions ¡this means “wanderer”

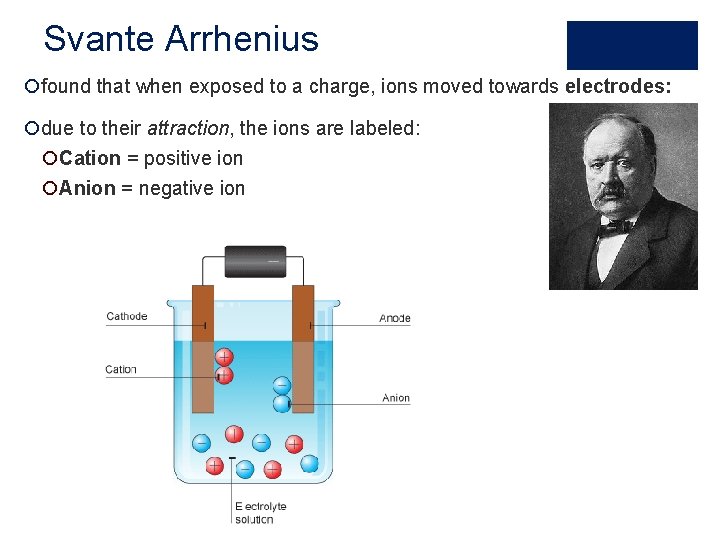
Svante Arrhenius ¡found that when exposed to a charge, ions moved towards electrodes: ¡due to their attraction, the ions are labeled: ¡Cation = positive ion ¡Anion = negative ion

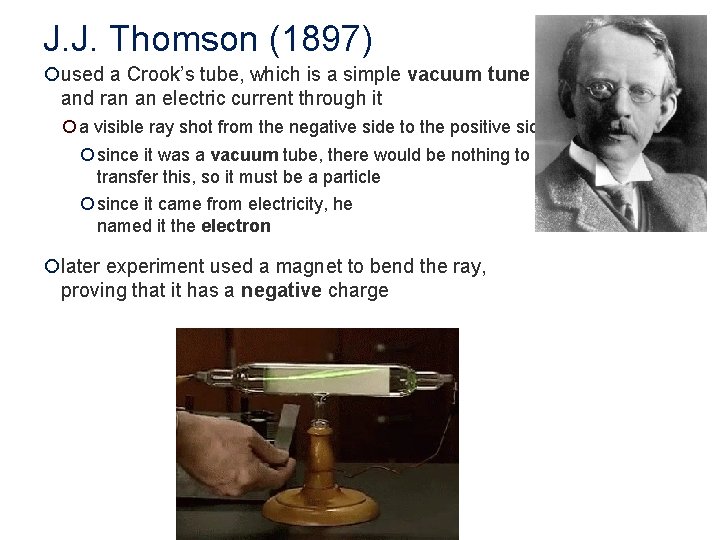
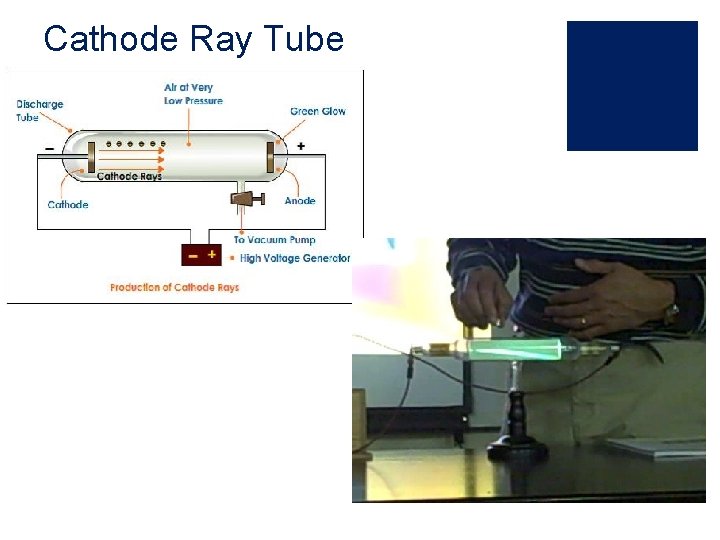
J. J. Thomson (1897) ¡used a Crook’s tube, which is a simple vacuum tune and ran an electric current through it ¡ a visible ray shot from the negative side to the positive side ¡ since it was a vacuum tube, there would be nothing to transfer this, so it must be a particle ¡ since it came from electricity, he named it the electron ¡later experiment used a magnet to bend the ray, proving that it has a negative charge

Cathode Ray Tube

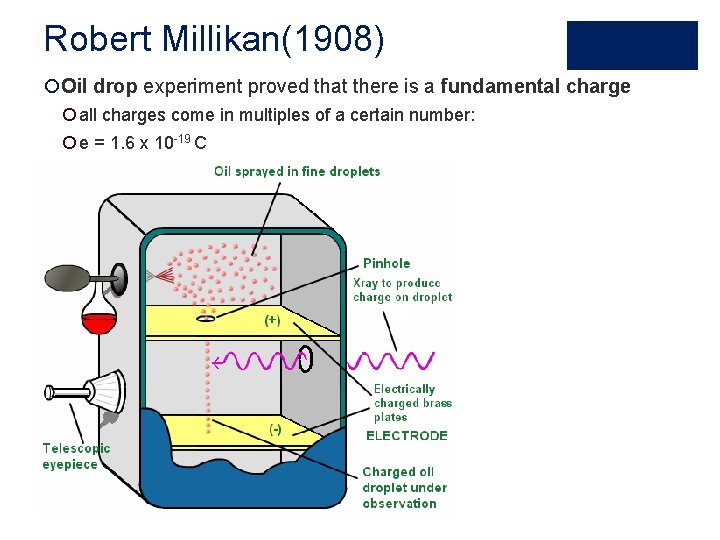
Robert Millikan(1908) ¡Oil drop experiment proved that there is a fundamental charge ¡ all charges come in multiples of a certain number: ¡ e = 1. 6 x 10 -19 C

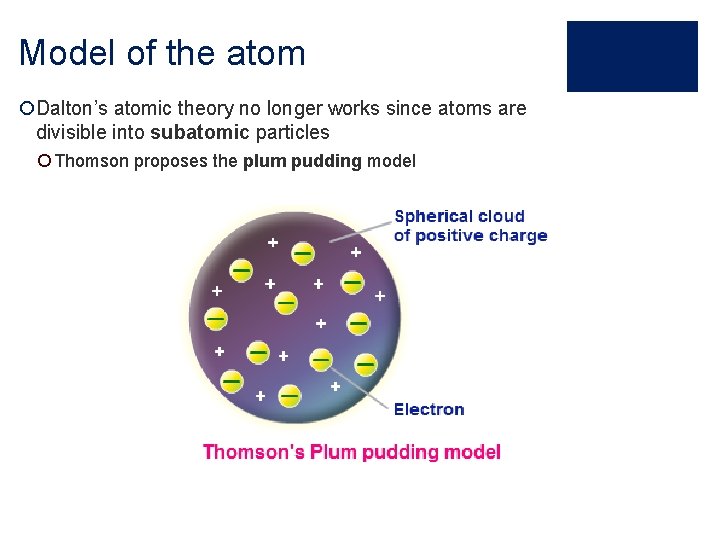
Model of the atom ¡Dalton’s atomic theory no longer works since atoms are divisible into subatomic particles ¡ Thomson proposes the plum pudding model

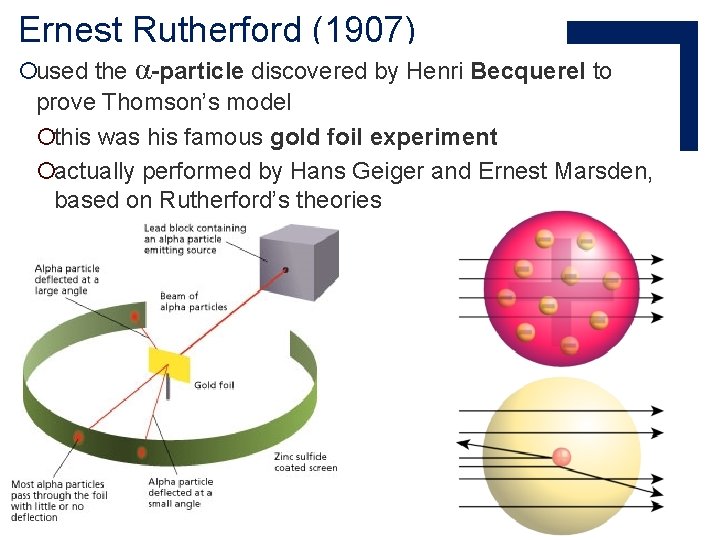
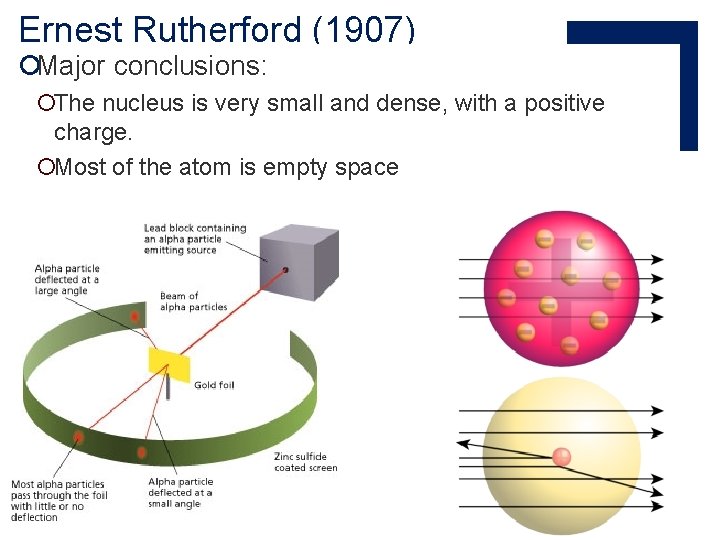
Ernest Rutherford (1907) ¡used the α-particle discovered by Henri Becquerel to prove Thomson’s model ¡this was his famous gold foil experiment ¡actually performed by Hans Geiger and Ernest Marsden, based on Rutherford’s theories

Ernest Rutherford (1907) ¡Major conclusions: ¡The nucleus is very small and dense, with a positive charge. ¡Most of the atom is empty space


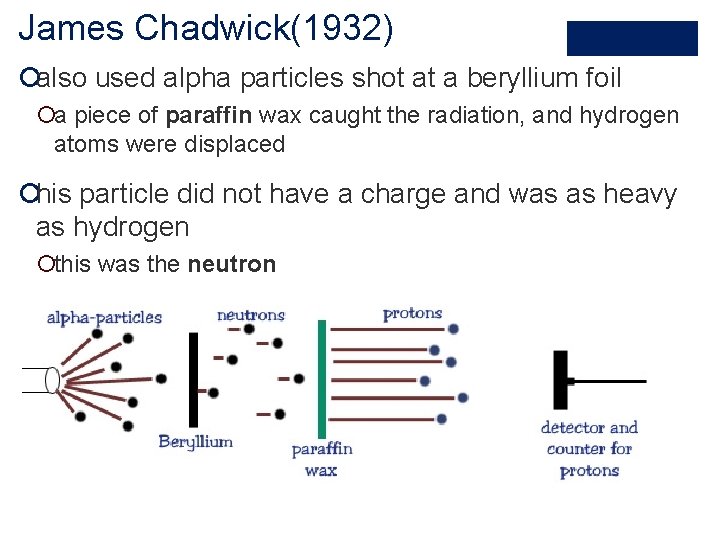
James Chadwick(1932) ¡also used alpha particles shot at a beryllium foil ¡a piece of paraffin wax caught the radiation, and hydrogen atoms were displaced ¡his particle did not have a charge and was as heavy as hydrogen ¡this was the neutron

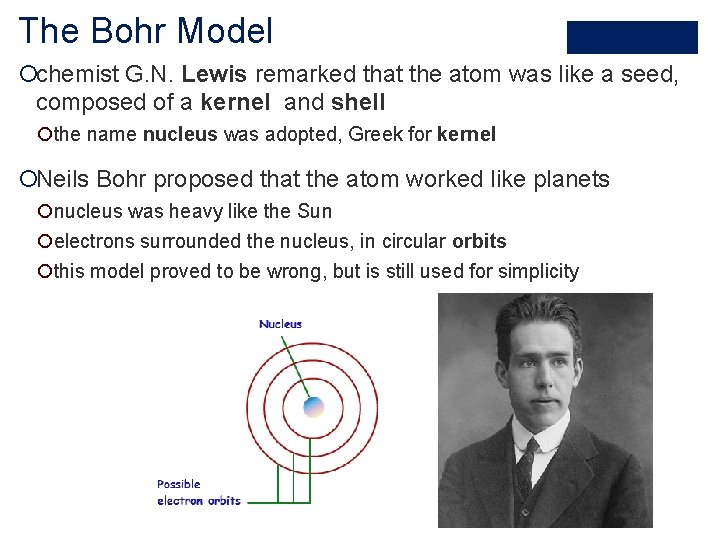
The Bohr Model ¡chemist G. N. Lewis remarked that the atom was like a seed, composed of a kernel and shell ¡the name nucleus was adopted, Greek for kernel ¡Neils Bohr proposed that the atom worked like planets ¡nucleus was heavy like the Sun ¡electrons surrounded the nucleus, in circular orbits ¡this model proved to be wrong, but is still used for simplicity

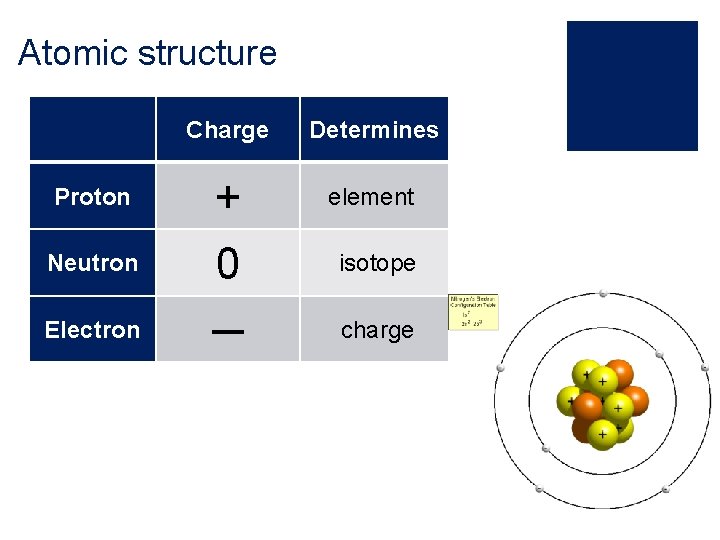
Atomic structure Charge Determines Proton + element Neutron 0 isotope Electron ─ charge

Isotopes ¡Meaning “same place” ¡Refers to location on periodic table ¡ Elements have same protons, but different neutrons ¡Usually expressed as a mass numbers ¡ the sum of protons plus neutrons Ex Carbon-14 is used in dating artifacts and very old samples. neutrons does carbon 14 have? How many

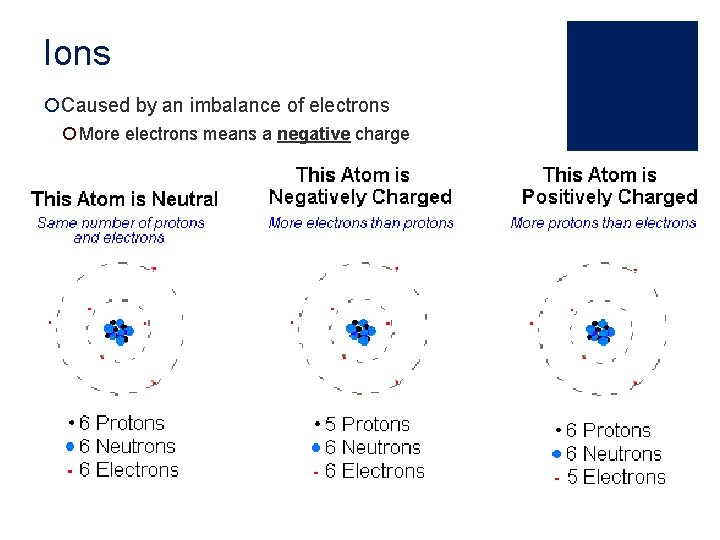
Ions ¡Caused by an imbalance of electrons ¡ More electrons means a negative charge

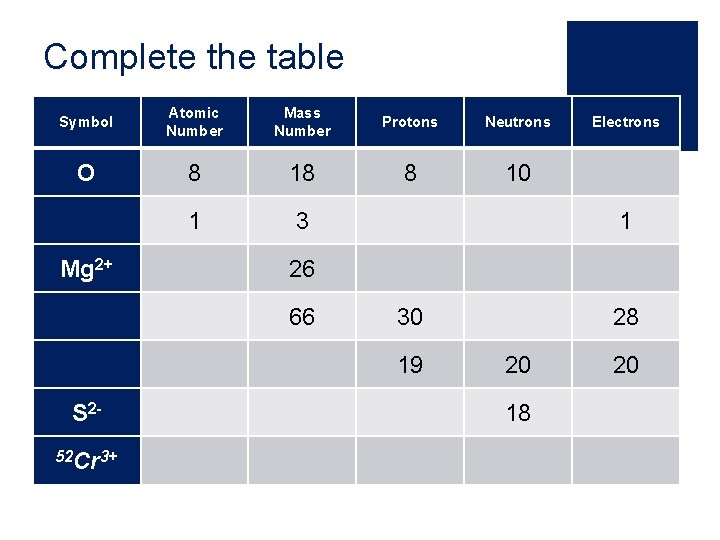
Complete the table Symbol Atomic Number Mass Number Protons Neutrons O 8 18 8 10 1 3 Mg 2+ 1 26 66 30 19 S 252 Cr 3+ Electrons 28 20 18 20