Atomic Structure Review Isotopes Atomic Structure n The



- Slides: 14

Atomic Structure Review Isotopes

Atomic Structure n The central region of each atom is called the……… Nucleus 2

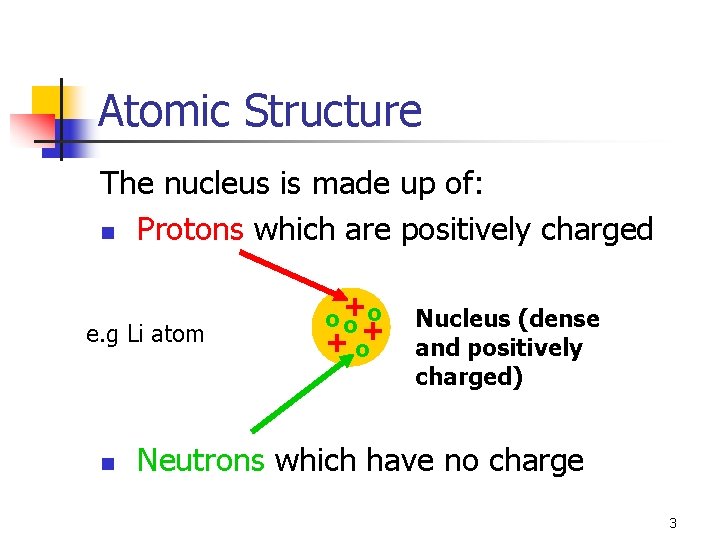
Atomic Structure The nucleus is made up of: n Protons which are positively charged e. g Li atom n o o+ o+ +o Nucleus (dense and positively charged) Neutrons which have no charge 3

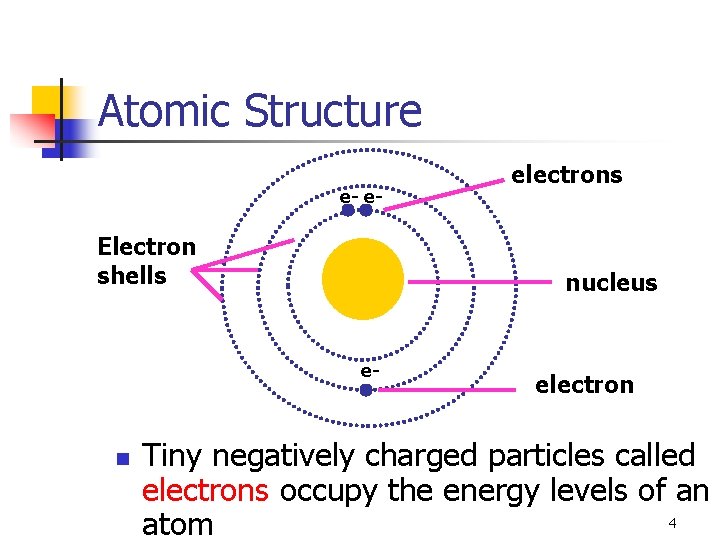
Atomic Structure e- e- Electron shells nucleus e- n electrons electron Tiny negatively charged particles called electrons occupy the energy levels of an 4 atom

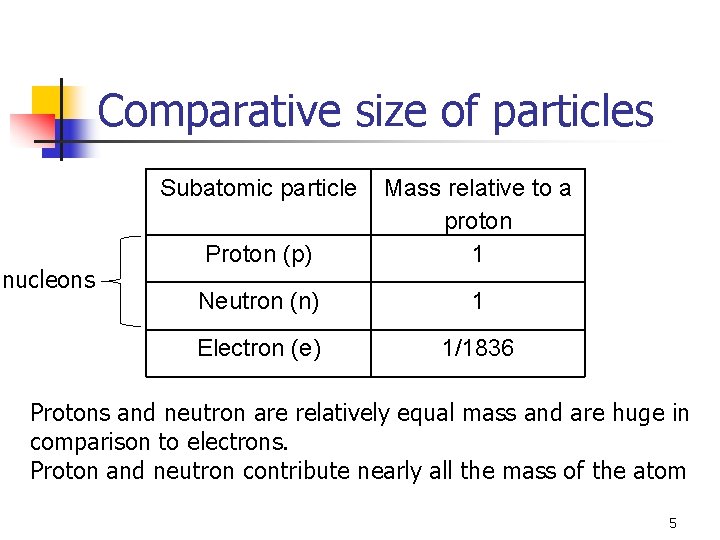
Comparative size of particles Subatomic particle nucleons Proton (p) Mass relative to a proton 1 Neutron (n) 1 Electron (e) 1/1836 Protons and neutron are relatively equal mass and are huge in comparison to electrons. Proton and neutron contribute nearly all the mass of the atom 5

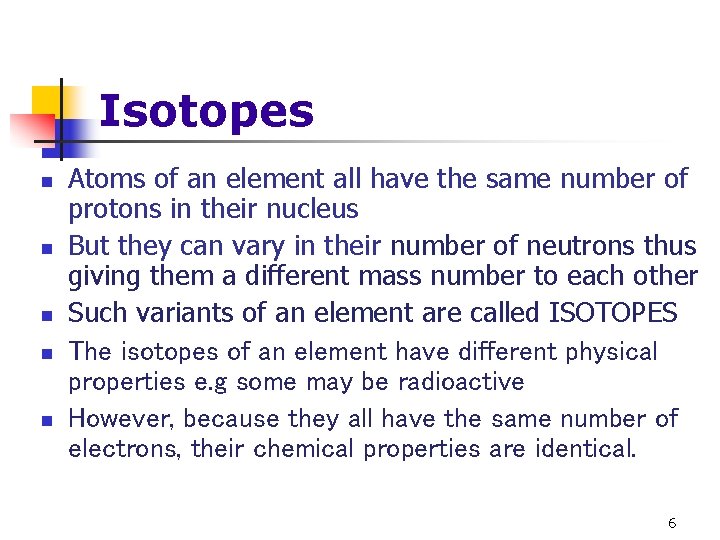
Isotopes n n n Atoms of an element all have the same number of protons in their nucleus But they can vary in their number of neutrons thus giving them a different mass number to each other Such variants of an element are called ISOTOPES The isotopes of an element have different physical properties e. g some may be radioactive However, because they all have the same number of electrons, their chemical properties are identical. 6

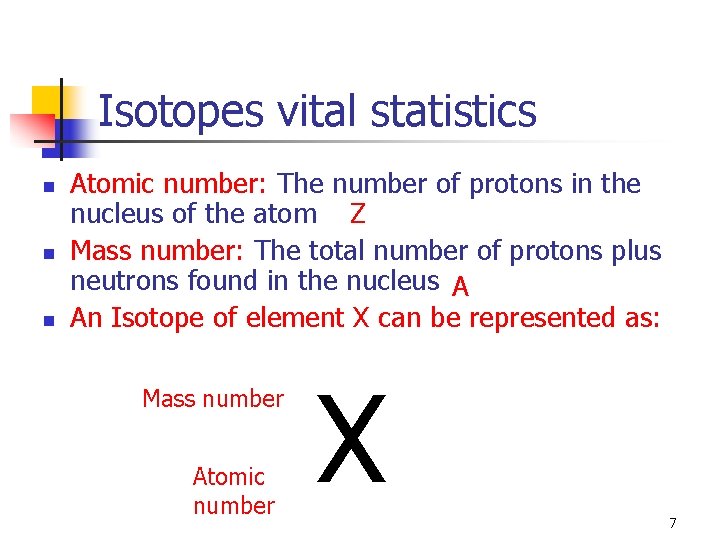
Isotopes vital statistics n n n Atomic number: The number of protons in the nucleus of the atom Z Mass number: The total number of protons plus neutrons found in the nucleus A An Isotope of element X can be represented as: Mass number Atomic number X 7

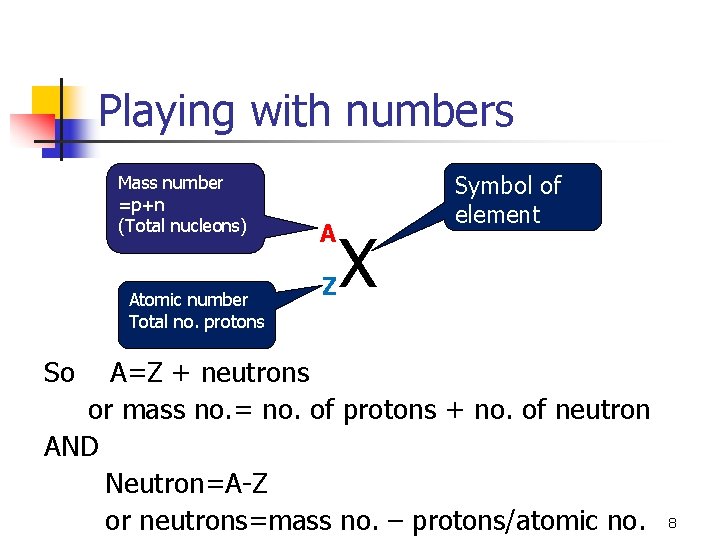
Playing with numbers Mass number =p+n (Total nucleons) Atomic number Total no. protons A Z X Symbol of element So A=Z + neutrons or mass no. = no. of protons + no. of neutron AND Neutron=A-Z or neutrons=mass no. – protons/atomic no. 8

Symbol for the most common isotope of Li Li Mass number 7 Atomic number 3 Lithium mass no= 7 atomic number= 3 Neutrons=4 (7 -3= 4) 9

F 19 9 Ca 40 20 Fluorine Mass no: 19 p: 9 n: (19 -9) 10 e: 9 Atomic no: 9 Calcium Mass no: 40 Atomic no: 20 p: 20 n: 20 e: 20

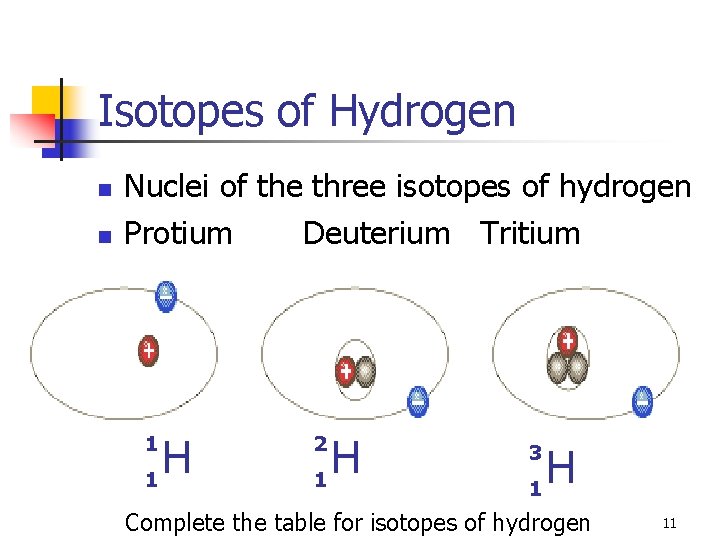
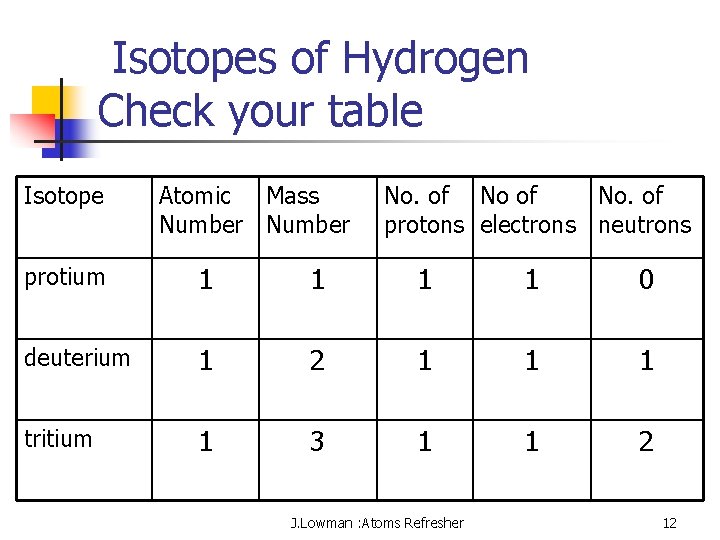
Isotopes of Hydrogen n n Nuclei of the three isotopes of hydrogen Protium Deuterium Tritium H 1 1 H 1 2 H 1 3 Complete the table for isotopes of hydrogen 11

Isotopes of Hydrogen Check your table Isotope Atomic Mass Number No. of protons electrons neutrons protium 1 1 0 deuterium 1 2 1 1 1 tritium 1 3 1 1 2 J. Lowman : Atoms Refresher 12

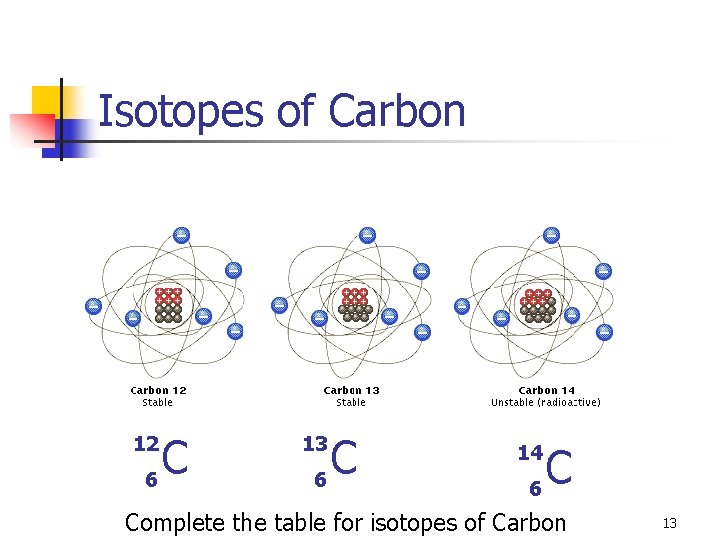
Isotopes of Carbon C 6 12 C 6 13 C 6 14 Complete the table for isotopes of Carbon 13

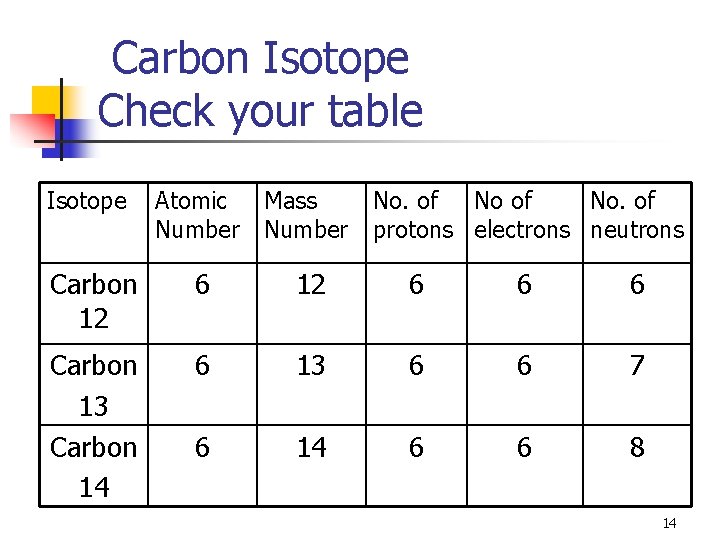
Carbon Isotope Check your table Isotope Atomic Number Mass Number No. of protons electrons neutrons Carbon 12 6 6 6 Carbon 13 Carbon 14 6 13 6 6 7 6 14 6 6 8 14