Isotopes and Radioactivity Isotopes Isotopes are atoms of





- Slides: 10

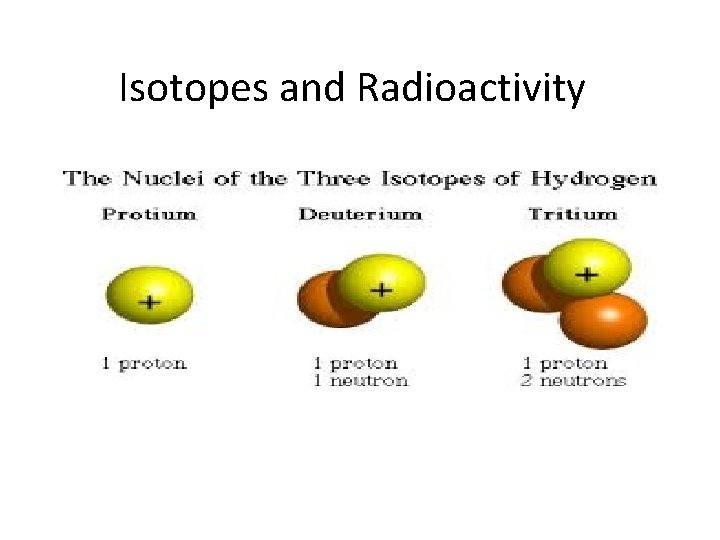
Isotopes and Radioactivity

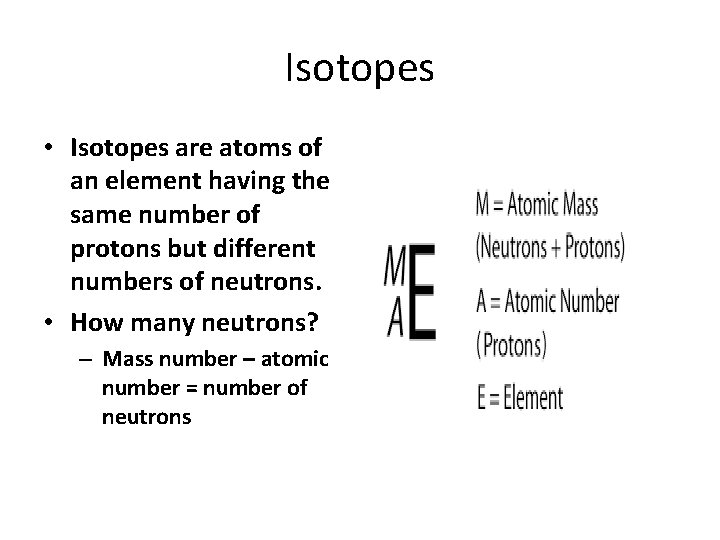
Isotopes • Isotopes are atoms of an element having the same number of protons but different numbers of neutrons. • How many neutrons? – Mass number – atomic number = number of neutrons

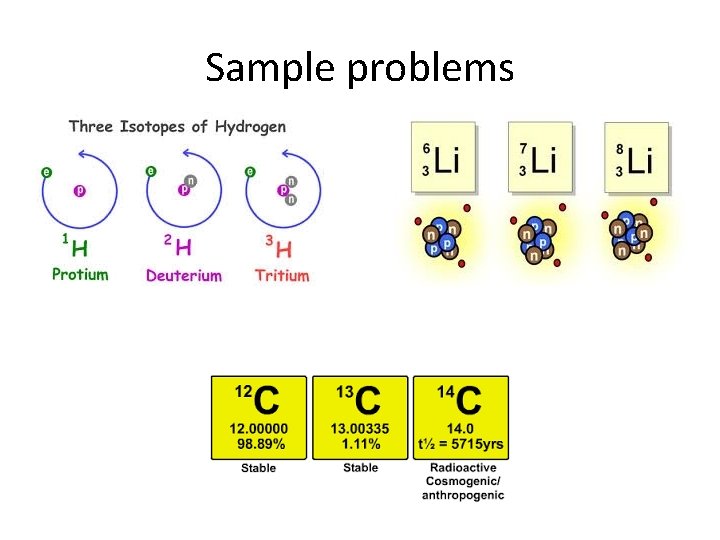
Sample problems

What is radioactive decay? • This occurs when an unstable atomic nucleus changes into another nucleus by emitting one or more particles and energy • A nucleus that is unstable is radioactive • Radioactive nuclei have found many uses in science and medicine – Iron-59 (an isotope) can be injected into a person’s bloodstream to show blood circulation – pg 303

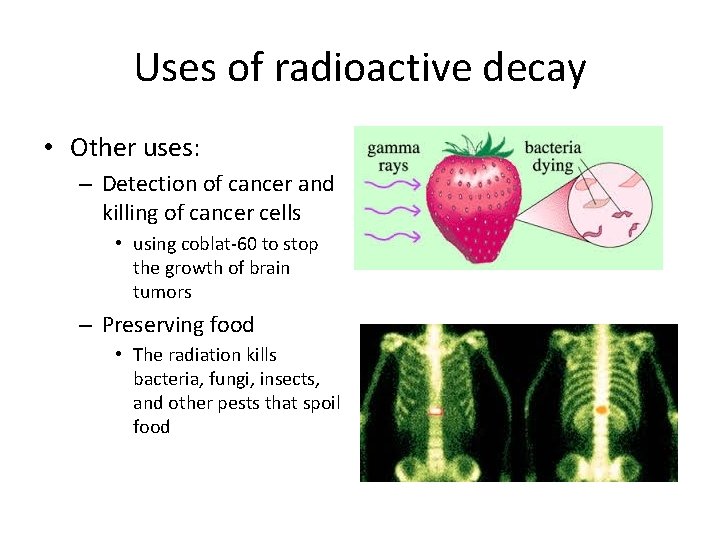
Uses of radioactive decay • Other uses: – Detection of cancer and killing of cancer cells • using coblat-60 to stop the growth of brain tumors – Preserving food • The radiation kills bacteria, fungi, insects, and other pests that spoil food

Discovery • 1896, Antoine-Henri Bequerel -> uranium • Marie Curie separated radioactive elements in uranium mineral – With her husband Pierre, discovered polonium and radium • Most radioactive elements are heavier elements, near the bottom of the periodic table

Half-Life • Uranium is still found in nature because of how long it takes to decay • Half-life is the time it takes for a sample of radioactive isotope to decay to half its original mass • EXAMPLES: – Uranium-235 takes 713 million years

How are elements discovered and named? • Some elements are not found in nature (or found in very small amounts) • Synthetic elements are radioactive elements made by scientists in labs or created during nuclear reactions • Technetium (43) was the first synthetic element • Visualizing Synthetic elements pg 308

Discovery • First they must be discovered and officially confirmed by International Union or Pure and Applied Chemistry and the International Union of Pure and Applied Physics – Write a paper – Experts review the work to support the claims made – Set of rules: must be able to be repeated, following scientific principles, show distinct chemical/physical properties

Naming • Scientists who discover the elements get to name them • They can name after people they want to honor or places of birth
 Antigentest åre
Antigentest åre Atoms and radioactivity
Atoms and radioactivity Isotopes pogil
Isotopes pogil Periodic table of elements regents
Periodic table of elements regents Natural and artificial radioactivity
Natural and artificial radioactivity Natural and artificial radioactivity
Natural and artificial radioactivity Natural and artificial radioactivity
Natural and artificial radioactivity Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Becquerel discovery of radioactivity
Becquerel discovery of radioactivity Who discovered radioactivity
Who discovered radioactivity Are nuclear power plants fission or fusion
Are nuclear power plants fission or fusion