ISOTOPES PG 27 ISOTOPES ISOTOPES ARE ATOMS OF











- Slides: 16

ISOTOPES PG. 27

ISOTOPES • ISOTOPES ARE ATOMS OF THE SAME ELEMENT THAT HAVE DIFFERENT NUMBERS OF NEUTRONS • TWO ISOTOPES OF AN ELEMENT WILL HAVE THE SAME ATOMIC NUMBER (# OF PROTONS) BUT DIFFERENT MASS NUMBERS WHAT IS THE MASS NUMBER TO THE LEFT? WHAT IS THE MASS NUMBER TO THE RIGHT? WHAT IS THE ATOMIC NUMBER TO THE LEFT? WHAT IS THE ATOMIC NUMBER TO THE RIGHT?

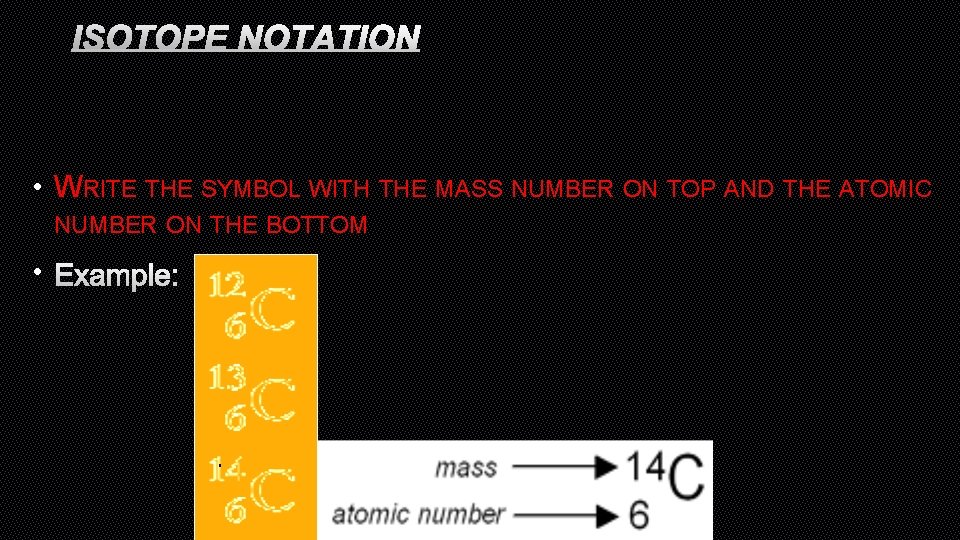
ISOTOPE NOTATION • WRITE THE SYMBOL WITH THE MASS NUMBER ON TOP AND THE ATOMIC NUMBER ON THE BOTTOM • EXAMPLE:

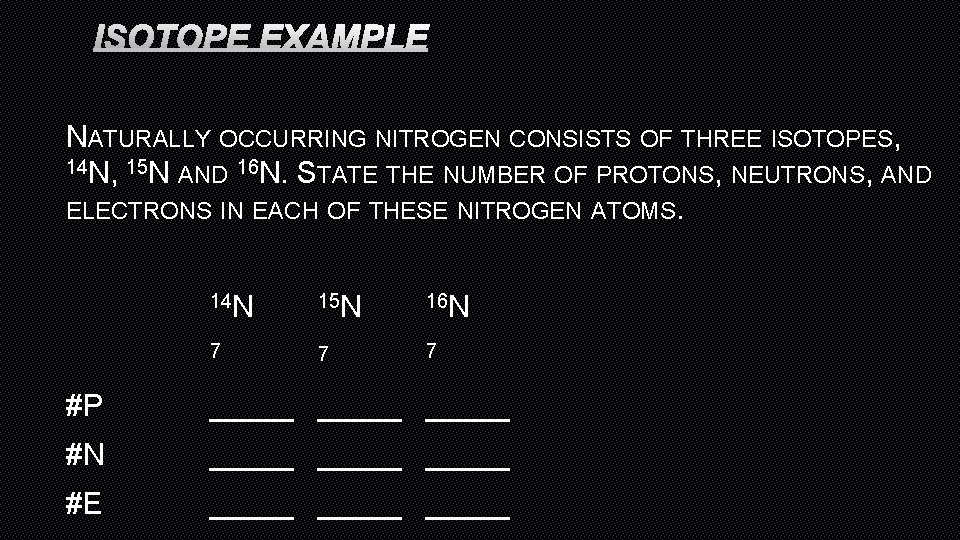
ISOTOPE EXAMPLE NATURALLY OCCURRING NITROGEN CONSISTS OF THREE ISOTOPES, 14 N, 15 N AND 16 N. STATE THE NUMBER OF PROTONS, NEUTRONS, AND ELECTRONS IN EACH OF THESE NITROGEN ATOMS. 14 N 15 N 16 N 7 7 7 #P _____ #N _____ #E _____

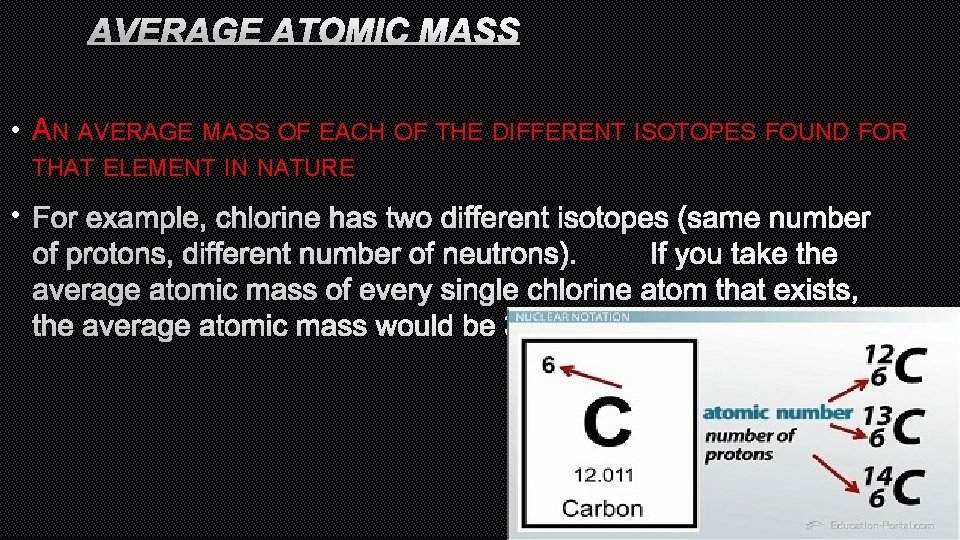
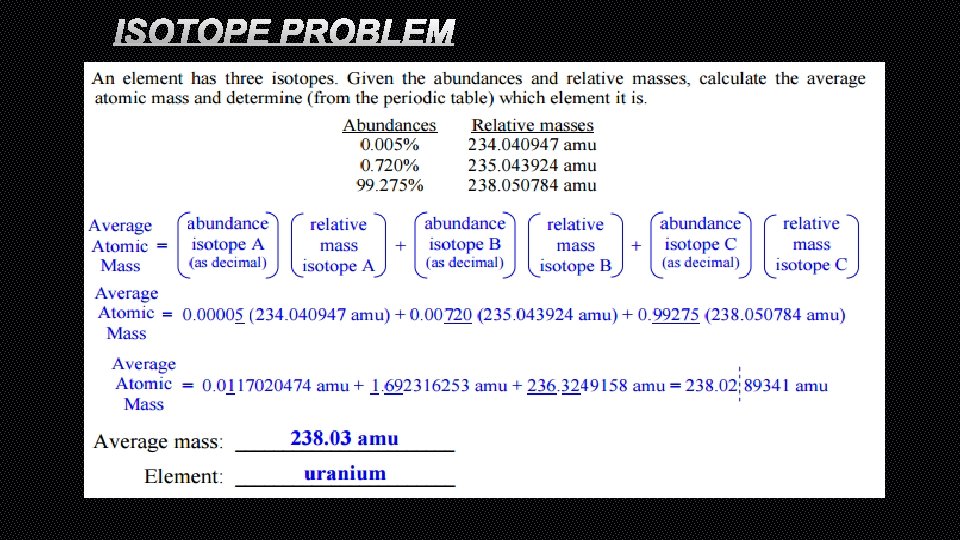
AVERAGE ATOMIC MASS • AN AVERAGE MASS OF EACH OF THE DIFFERENT ISOTOPES FOUND FOR THAT ELEMENT IN NATURE • FOR EXAMPLE, CHLORINE HAS TWO DIFFERENT ISOTOPES (SAME NUMBER OF PROTONS, DIFFERENT NUMBER OF NEUTRONS). IF YOU TAKE THE AVERAGE ATOMIC MASS OF EVERY SINGLE CHLORINE ATOM THAT EXISTS, THE AVERAGE ATOMIC MASS WOULD BE 35. 453 AMU

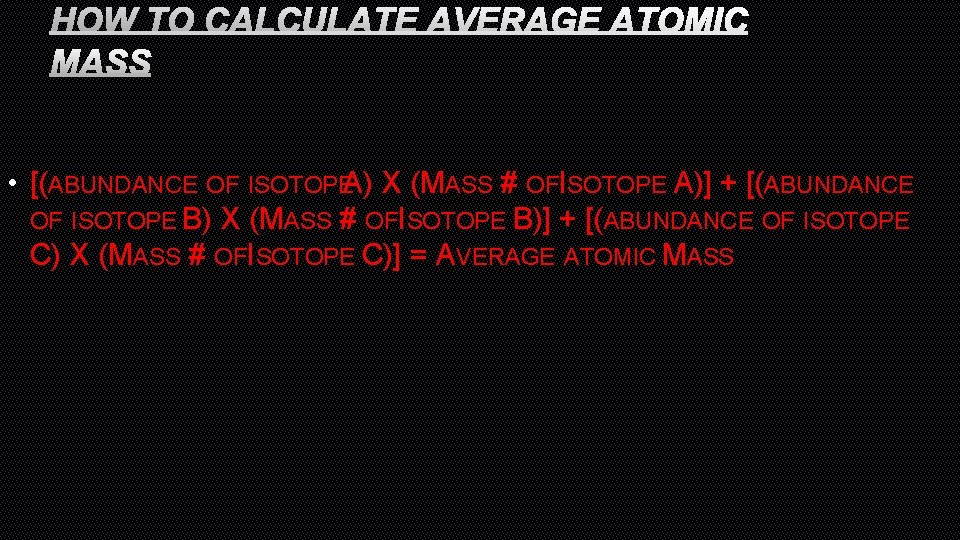
HOW TO CALCULATE AVERAGE ATOMIC MASS • [(ABUNDANCE OF ISOTOPEA) X (MASS # OFISOTOPE A)] + [(ABUNDANCE OF ISOTOPE B) X (MASS # OFISOTOPE B)] + [(ABUNDANCE OF ISOTOPE C) X (MASS # OFISOTOPE C)] = AVERAGE ATOMIC MASS

ISOTOPE PROBLEM



RADIOACTIVITY

RADIOACTIVITY • SOME ELEMENTS WILL CHANGE (DECAY) INTO ANOTHER ELEMENT. EX: RADON GAS TURNS INTO LEAD. • THESE ELEMENTS ARE CALLED: RADIOACTIVE • WHEN THESE ELEMENTS DECAY THEIRNUCLEUS CHANGES INTO ANOTHER ELEMENT AND THEY GIVE OFF RADIATION

TYPES OF RADIATION 3 TYPES OF RADIATION 1. ALPHA DECAY ( Α ): • WEAKEST –CAN BE STOPPED BY A PIECE OF PAPER. 2. BETA DECAY ( Β ): • 2 ND STRONGEST – CAN BE STOPPED BY ALUMINUMFOIL. 3. GAMMA RAYS ( Γ ): • STOPPED BY 5 CM OF LEAD.

HALF-LIFE • EACH RADIOACTIVE ELEMENT HAS A CERTAINHALF – LIFE. • HALF – LIFE: THE AMOUNT OF TIME IT TAKES FOR HALF OF THE ELEMENT TO DECAY (CHANGE).

HALF-LIFE EXAMPLE • YOU HAVE 8 M&M’S. IF THE HALF-LIFE OFM&M’S IS 1 MINUTE, HOW MANY WILL BE LEFT AFTER 3 MINUTES? NUMBER OF M&M’S TIME 8 0 4 1 2 2 _ 3

HALF-LIFE EXAMPLE IF YOU HAD 100 G OF A RADIOACTIVESUBSTANCE THAT HAS A HALFLIFE OF 4 DAYS, HOWMUCH WOULD BE LEFT AFTER 8 DAYS? RADIOACTIVE SUBSTANCE DAYS 100 G 0 50 G 4 ____ 8

HALF-LIFE EXAMPLE IF YOU HAD 1000 G OF A RADIOACTIVE SUBSTANCE THAT HAS A HALF-LIFE OF 25 YEARS, HOWMUCH WOULD BE LEFT AFTER 100 YEARS? RADIOACTIVE SUBSTANCE 1000 G 500 G 250 G 125 G _____ YEARS 0 25 50 75 100