SNC 1 D Isotopes Isotopes Isotopes are atoms








- Slides: 20

SNC 1 D Isotopes

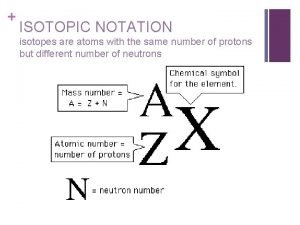
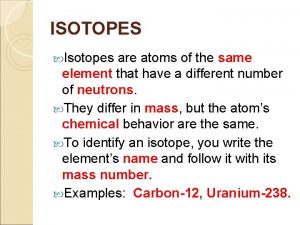
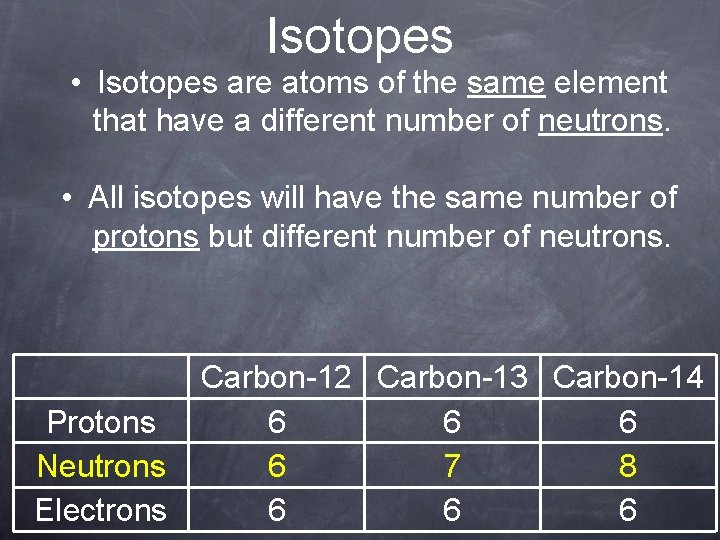
Isotopes • Isotopes are atoms of the same element that have a different number of neutrons. • All isotopes will have the same number of protons but different number of neutrons. Protons Neutrons Electrons Carbon-12 Carbon-13 Carbon-14 6 6 7 8 6 6 6

Neutron + Electrons Nucleus + + + Nucleus Carbon-12 Neutrons 6 Protons 6 Electrons 6 + + Nucleus Proton + + Neutron Electrons + + Carbon-14 Neutrons 8 Protons 6 Electrons 6 Nucleus

Isotopes • Therefore the mass number of each isotope will be different. • Isotopes of the same element have the same chemical properties but could have slightly different physical properties.

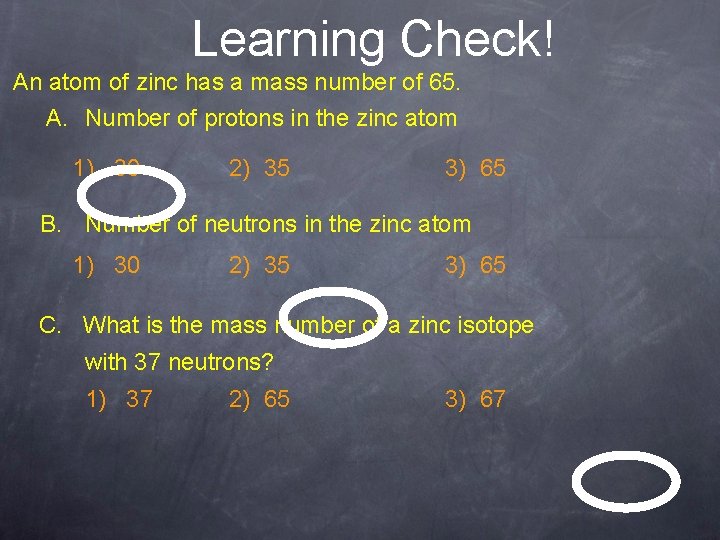
Learning Check! An atom of zinc has a mass number of 65. A. Number of protons in the zinc atom 1) 30 2) 35 3) 65 B. Number of neutrons in the zinc atom 1) 30 2) 35 3) 65 C. What is the mass number of a zinc isotope with 37 neutrons? 1) 37 2) 65 3) 67

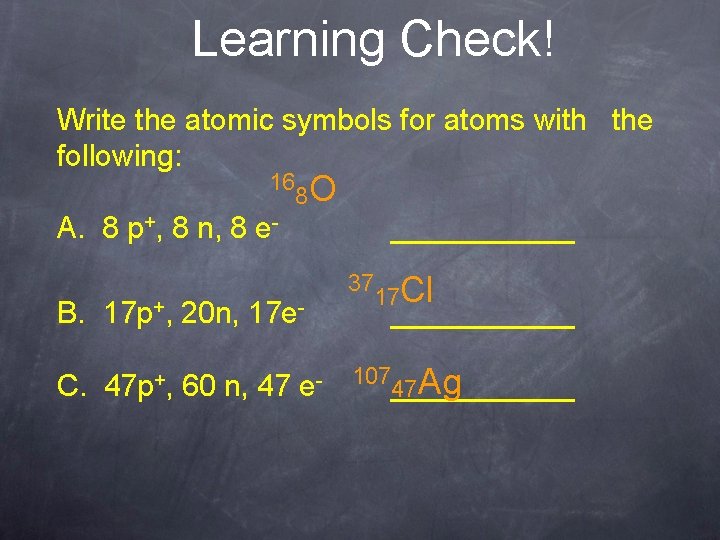
Learning Check! Write the atomic symbols for atoms with the following: 16 8 O A. 8 p+, 8 n, 8 e. B. 17 p+, C. 47 p+, 20 n, ______ 17 e- 60 n, 47 e- 37 17 Cl ___________ 47 Ag 107

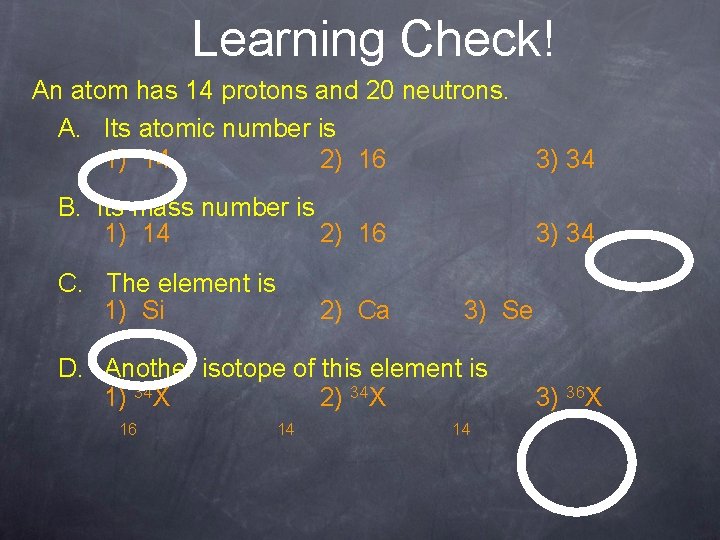
Learning Check! An atom has 14 protons and 20 neutrons. A. Its atomic number is 1) 14 2) 16 3) 34 B. Its mass number is 1) 14 2) 16 C. The element is 1) Si 2) Ca 3) 34 3) Se D. Another isotope of this element is 1) 34 X 2) 34 X 16 14 14 3) 36 X

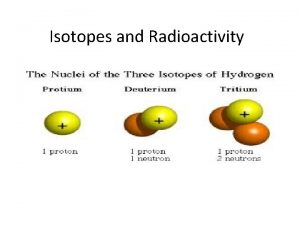
Isotopes of Hydrogen Mass number Standard atomic notation Isotope # protons # electrons # neutrons Protium 1 1 0 1 1 1 H Deuterium 1 1 1 2 2 1 H Tritium 1 1 2 3 3 1 H

Isotopes Notation • Isotopes can be indicated by writing the symbol or name of the element followed by a dash and the mass number (e. g. Carbon-14 or C-14) Isotopes of chlorine 35 17 Cl chlorine - 35 37 17 Cl chlorine - 37 Practice: How many neutrons are in each of the following isotopes? • Cobalt-60 • Iodine-131 • Uranium-235

Uses of Isotopes • Carbon-14 exists in a set ratio with Carbon-12 • When the organism dies, C 14 decays, but C-12 does not • The percentage of C-14 decreases as the age of the dead organism increases. This percentage is used to estimate the age of the organism.

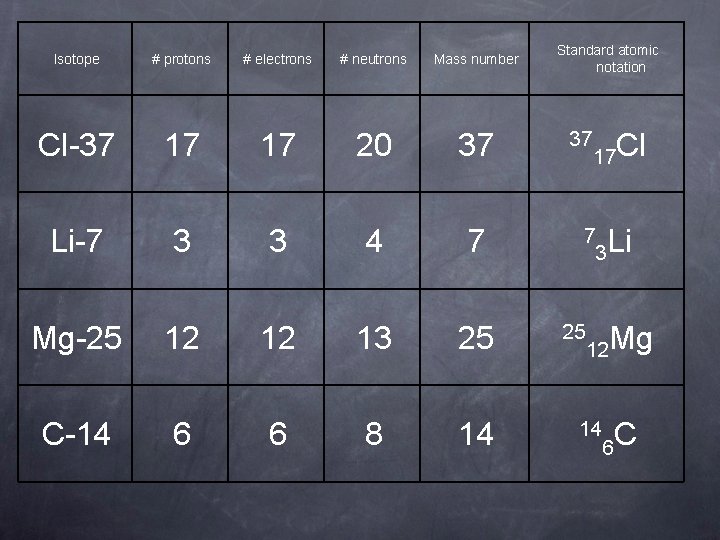
Isotope # protons # electrons # neutrons Mass number Cl-37 17 17 20 37 Li-7 3 3 4 7 Mg-25 12 12 13 25 C-14 6 6 8 14 Standard atomic notation 37 17 Cl 7 25 3 Li 12 Mg 14 6 C

Masses of Atoms l A scale designed for atoms gives their small atomic masses in atomic mass units (amu) l An atom of 12 C was assigned an exact mass of 12. 00 amu l Relative masses of all other atoms was determined by comparing each to the mass of 12 C l An atom twice as heavy has a mass of 24. 00 amu. An atom half as heavy is 6. 00 amu.

Atomic Mass l Listed on the periodic table Na 22. 99 l Gives the mass of “average” atom of each element compared to 12 C l Average atom based on all the isotopes and their abundance % l Atomic mass is not a whole number

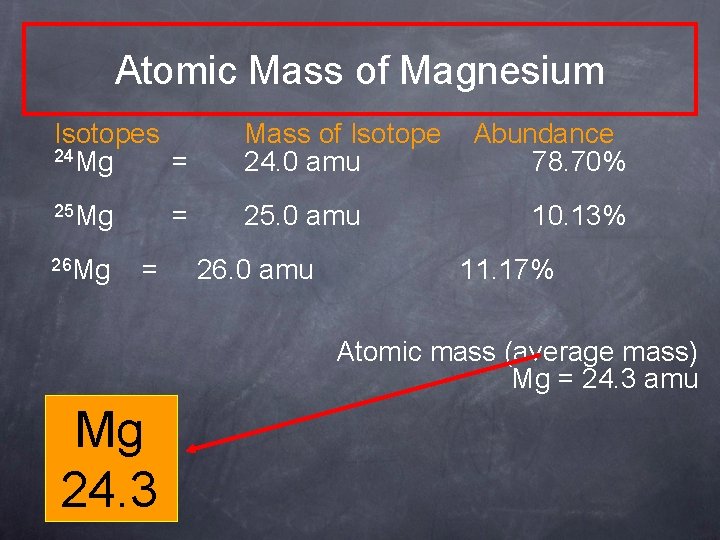
Atomic Mass of Magnesium Isotopes 24 Mg = Mass of Isotope 24. 0 amu 25 Mg 25. 0 amu 26 Mg = = 26. 0 amu Abundance 78. 70% 10. 13% 11. 17% Atomic mass (average mass) Mg = 24. 3 amu Mg 24. 3

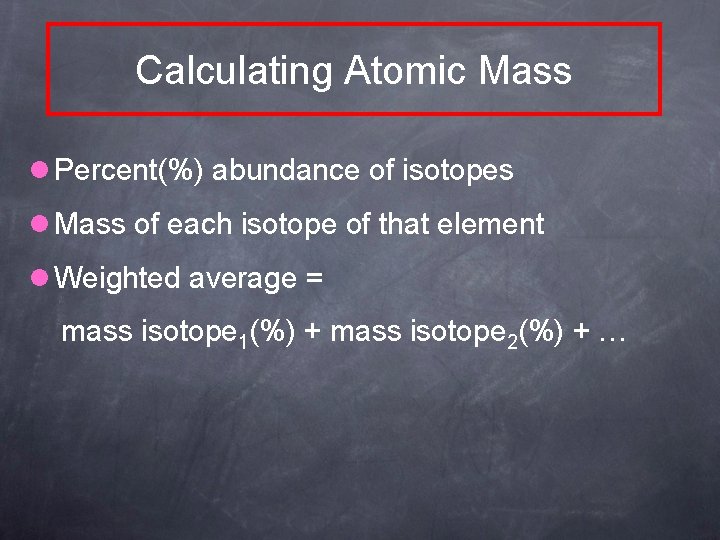
Calculating Atomic Mass l Percent(%) abundance of isotopes l Mass of each isotope of that element l Weighted average = mass isotope 1(%) + mass isotope 2(%) + …

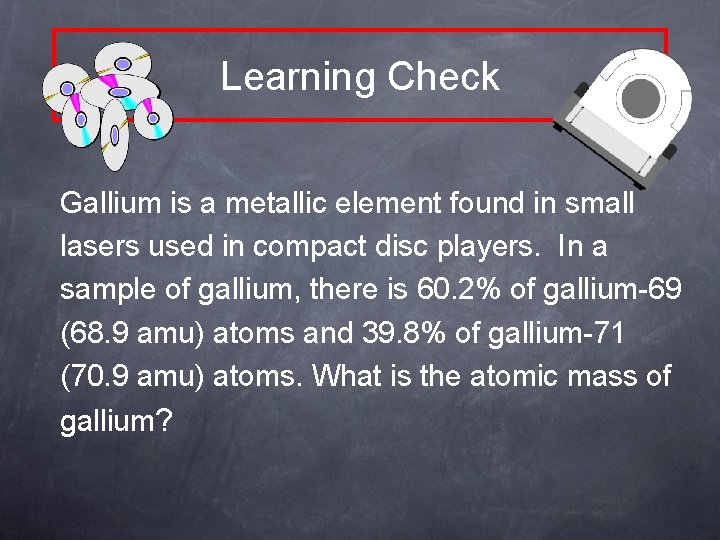
Learning Check Gallium is a metallic element found in small lasers used in compact disc players. In a sample of gallium, there is 60. 2% of gallium-69 (68. 9 amu) atoms and 39. 8% of gallium-71 (70. 9 amu) atoms. What is the atomic mass of gallium?

68. 9 amu x 0. 602 + 70. 9 amu x 0. 398 Atomic mass Ga = 69. 7 amu

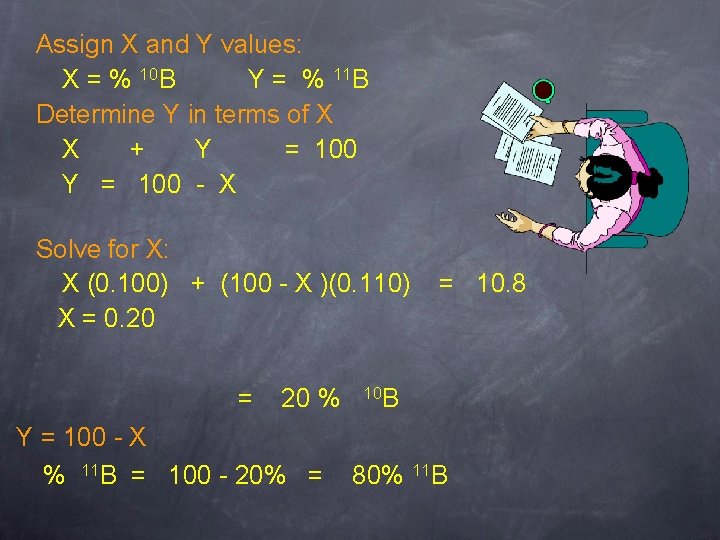
Finding An Isotopic Mass A sample of boron consists of 10 B (mass 10. 0 amu) and 11 B (mass 11. 0 amu). If the average atomic mass of B is 10. 8 amu, what is the % abundance of each boron isotope?

Assign X and Y values: X = % 10 B Y = % 11 B Determine Y in terms of X X + Y = 100 - X Solve for X: X (0. 100) + (100 - X )(0. 110) X = 0. 20 = 20 % Y = 100 - X % 11 B = 100 - 20% = = 10. 8 10 B 80% 11 B

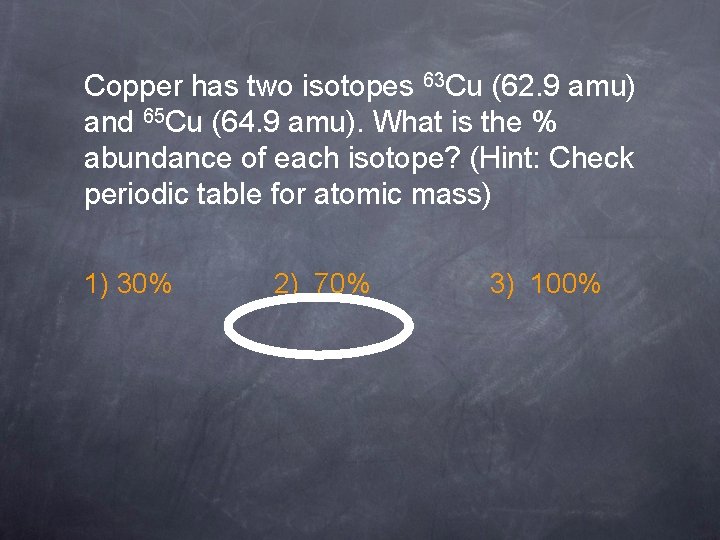
Copper has two isotopes 63 Cu (62. 9 amu) and 65 Cu (64. 9 amu). What is the % abundance of each isotope? (Hint: Check periodic table for atomic mass) 1) 30% 2) 70% 3) 100%
 Mikael ferm
Mikael ferm Isotopes pogil
Isotopes pogil Compared to atoms of metals, atoms of nonmetals generally
Compared to atoms of metals, atoms of nonmetals generally Rombencefalo
Rombencefalo Raquisis
Raquisis Snc-1
Snc-1 Paul mcenaney
Paul mcenaney Snc lajaa venissieux
Snc lajaa venissieux Hhps symbols
Hhps symbols Snc
Snc Hydrogen isotopes
Hydrogen isotopes Orbital diagram for rubidium
Orbital diagram for rubidium Isotopes radioactifs
Isotopes radioactifs Isotopes examples
Isotopes examples Subatomic heavyweights isotopes lesson 13
Subatomic heavyweights isotopes lesson 13 Isotope abundance formula
Isotope abundance formula Isotopes examples
Isotopes examples Isotopic notation
Isotopic notation Uses of radioactive isotopes in agriculture
Uses of radioactive isotopes in agriculture Hydrogen isotopes
Hydrogen isotopes Isotopes properties
Isotopes properties