Nonmetallic Elements and Their Compounds Chapter 22 1



- Slides: 32

Nonmetallic Elements and Their Compounds Chapter 22 1 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

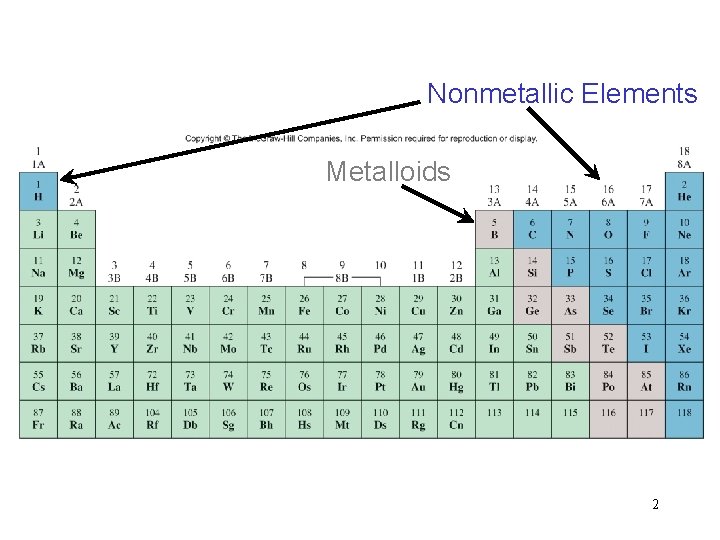
Nonmetallic Elements Metalloids 2

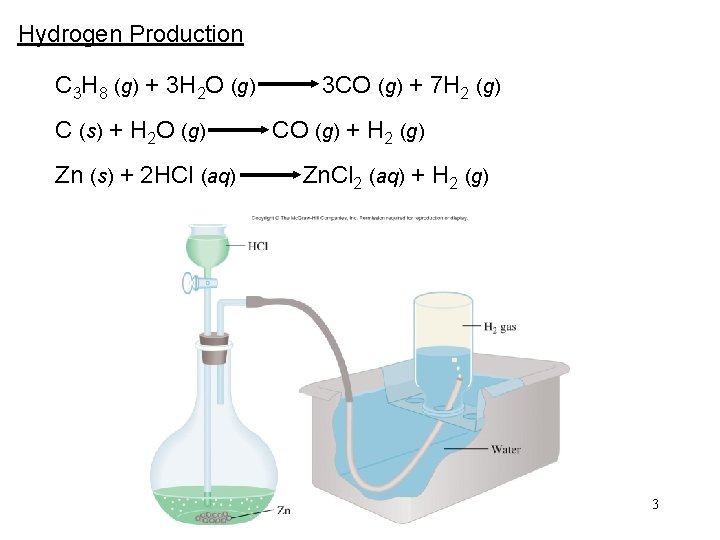
Hydrogen Production C 3 H 8 (g) + 3 H 2 O (g) 3 CO (g) + 7 H 2 (g) C (s) + H 2 O (g) CO (g) + H 2 (g) Zn (s) + 2 HCl (aq) Zn. Cl 2 (aq) + H 2 (g) 3

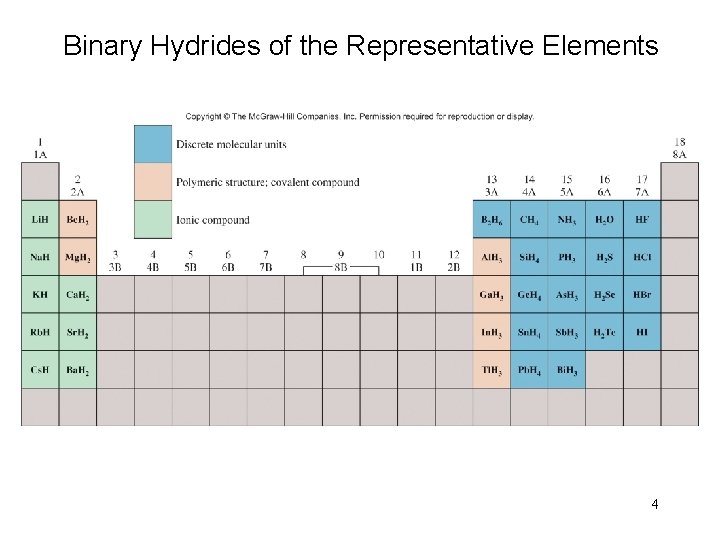
Binary Hydrides of the Representative Elements 4

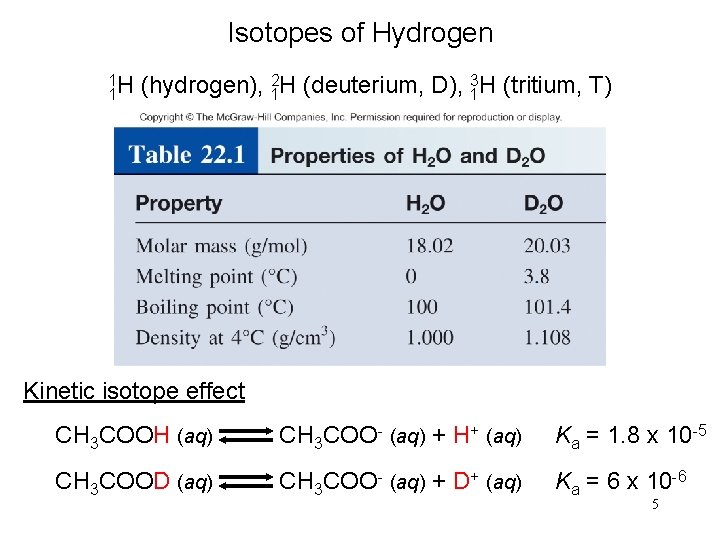
Isotopes of Hydrogen 1 2 3 1 H (hydrogen), 1 H (deuterium, D), 1 H (tritium, T) Kinetic isotope effect CH 3 COOH (aq) CH 3 COO- (aq) + H+ (aq) Ka = 1. 8 x 10 -5 CH 3 COOD (aq) CH 3 COO- (aq) + D+ (aq) Ka = 6 x 10 -6 5

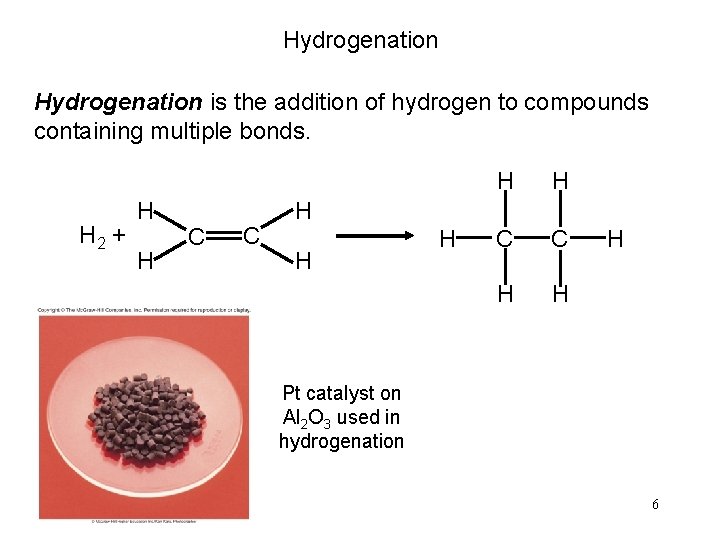
Hydrogenation is the addition of hydrogen to compounds containing multiple bonds. H 2 + H H C C H H H Pt catalyst on Al 2 O 3 used in hydrogenation 6

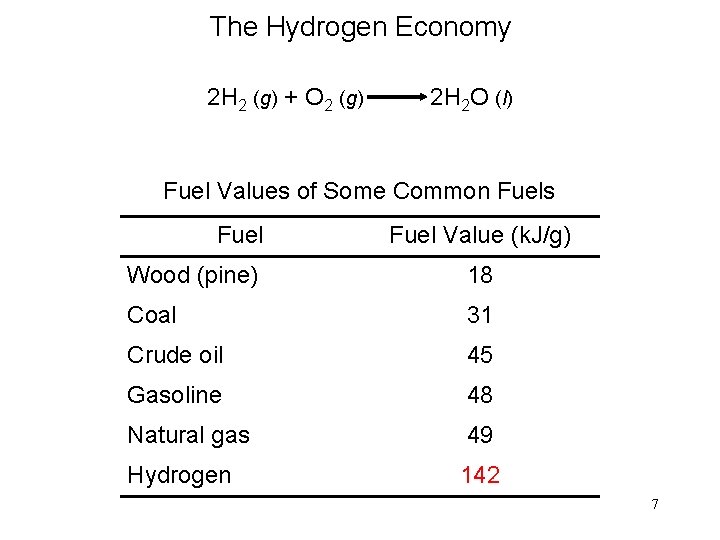
The Hydrogen Economy 2 H 2 (g) + O 2 (g) 2 H 2 O (l) Fuel Values of Some Common Fuels Fuel Value (k. J/g) Wood (pine) 18 Coal 31 Crude oil 45 Gasoline 48 Natural gas 49 Hydrogen 142 7

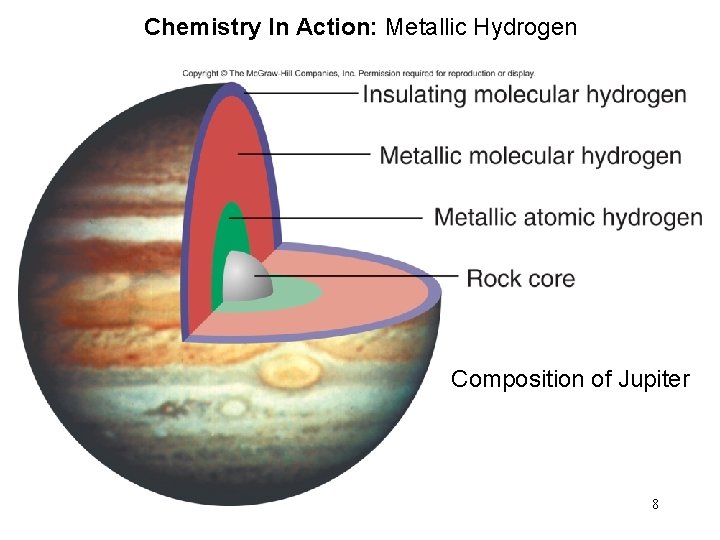
Chemistry In Action: Metallic Hydrogen Composition of Jupiter 8

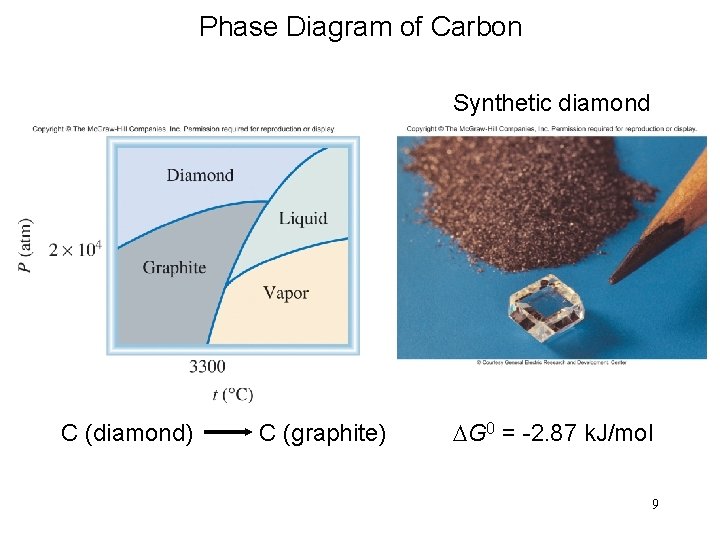
Phase Diagram of Carbon Synthetic diamond C (diamond) C (graphite) DG 0 = -2. 87 k. J/mol 9

Chemistry In Action: Synthetic Gas from Coal C (s) + H 2 O (g) CO (g) + H 2 (g) C (s) + 2 H 2 (g) CH 4 (g) CO (g) + 2 H 2 (g) CH 3 OH (l) 10

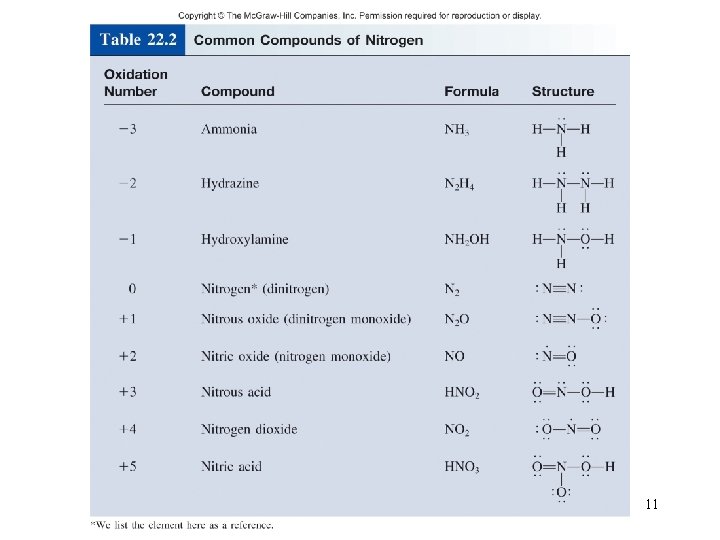
11

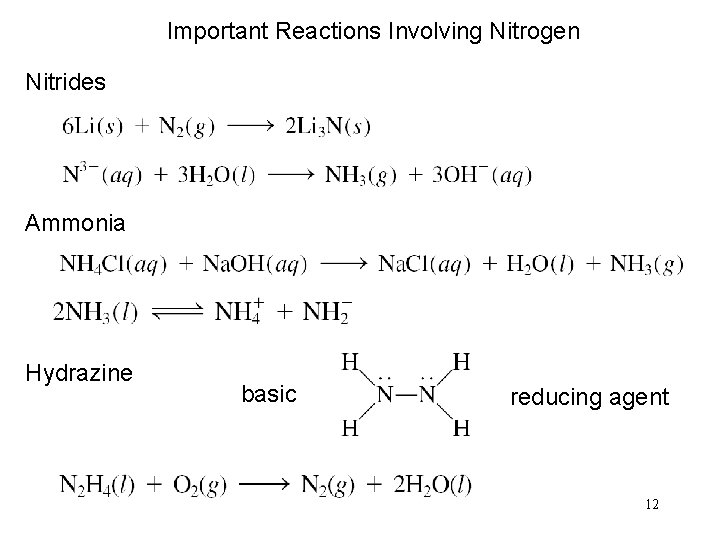
Important Reactions Involving Nitrogen Nitrides Ammonia Hydrazine basic reducing agent 12

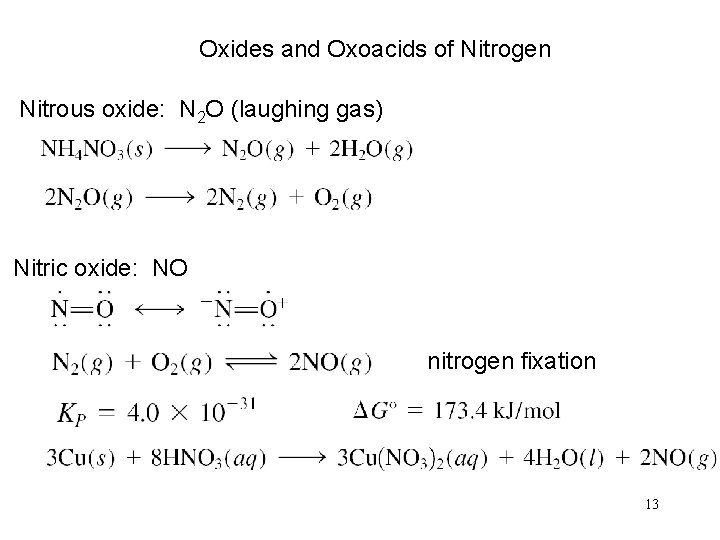
Oxides and Oxoacids of Nitrogen Nitrous oxide: N 2 O (laughing gas) Nitric oxide: NO nitrogen fixation 13

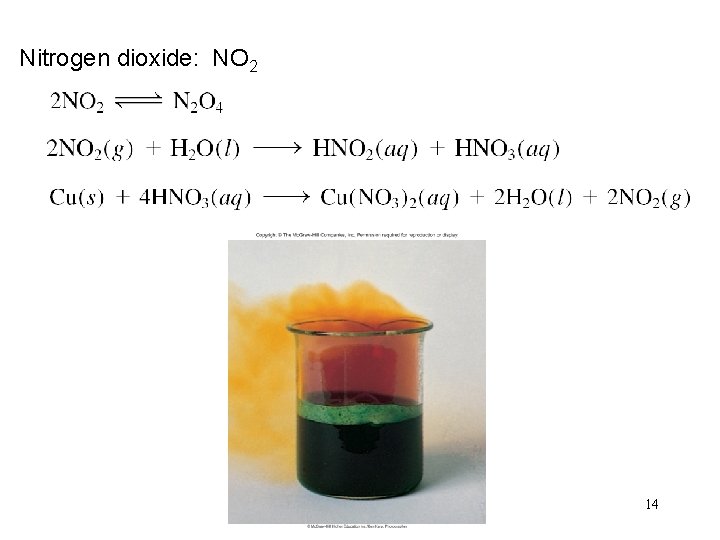
Nitrogen dioxide: NO 2 14

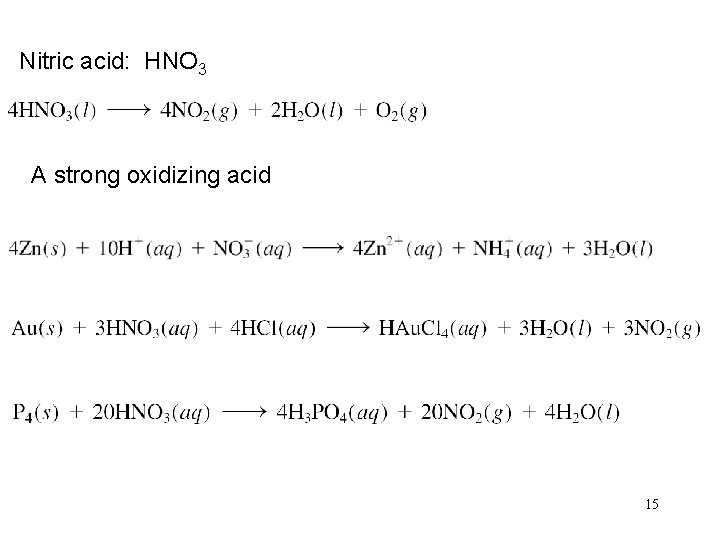
Nitric acid: HNO 3 A strong oxidizing acid 15

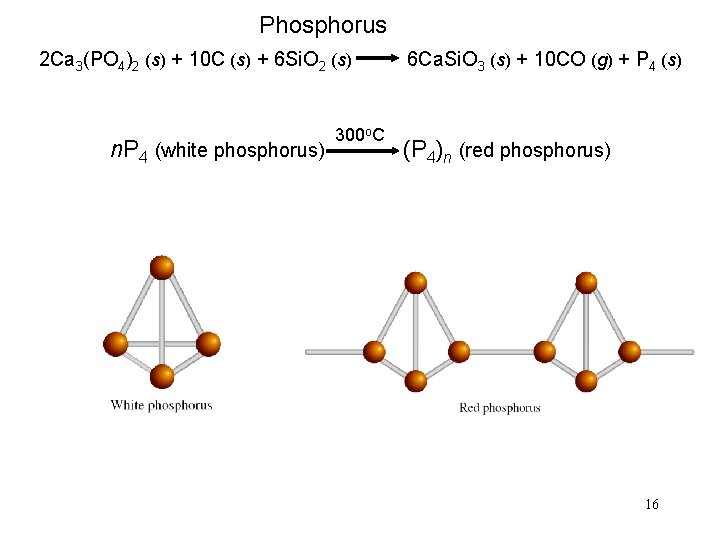
Phosphorus 2 Ca 3(PO 4)2 (s) + 10 C (s) + 6 Si. O 2 (s) 6 Ca. Si. O 3 (s) + 10 CO (g) + P 4 (s) 300 o. C n. P 4 (white phosphorus) (P 4)n (red phosphorus) 16

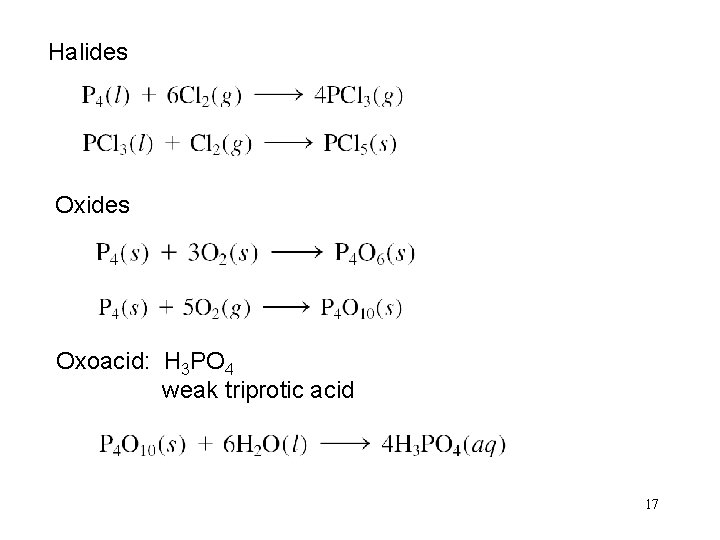
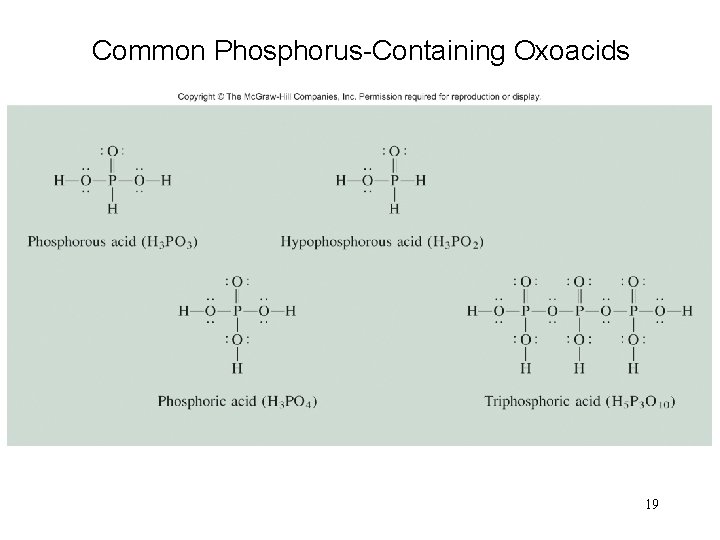
Halides Oxoacid: H 3 PO 4 weak triprotic acid 17

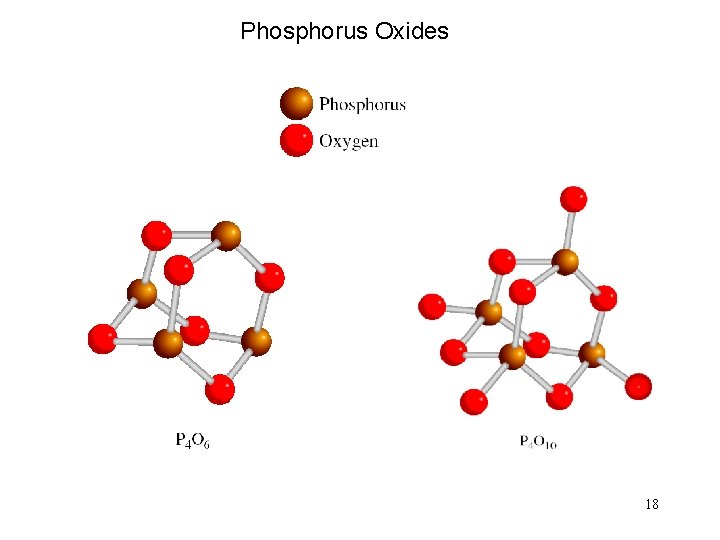
Phosphorus Oxides 18

Common Phosphorus-Containing Oxoacids 19

Chemistry In Action: Ammonium Nitrate–The Explosive Fertilizer At T > 2500 C NH 4 NO 3 (g) N 2 O (g) + 2 H 2 O (g) At T > 3000 C 2 NH 4 NO 3 (g) 2 N 2 (g) + 4 H 2 O (g) + O 2 (g) Alfred P. Murrah Building Bag of Fertilizer 20

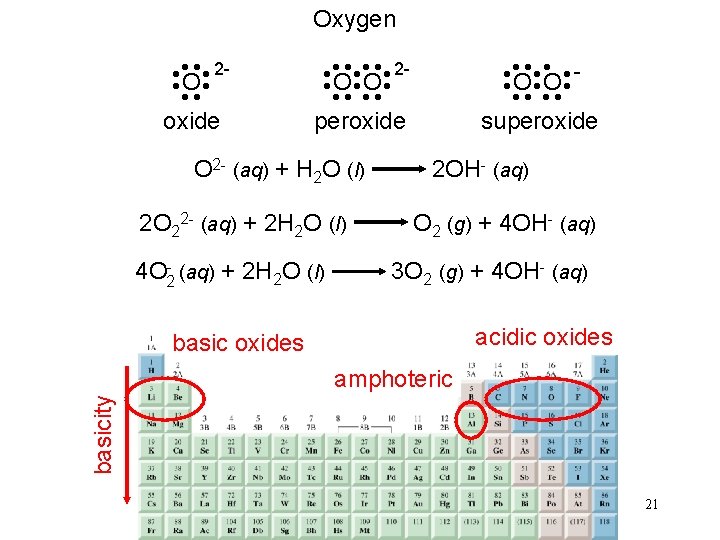
Oxygen • • • O O • • superoxide • • • • 2 O O • • peroxide • • • • 2 O • • oxide O 2 - (aq) + H 2 O (l) 2 OH- (aq) 2 O 22 - (aq) + 2 H 2 O (l) O 2 (g) + 4 OH- (aq) 4 O 2 - (aq) + 2 H 2 O (l) 3 O 2 (g) + 4 OH- (aq) acidic oxides basicity amphoteric 21

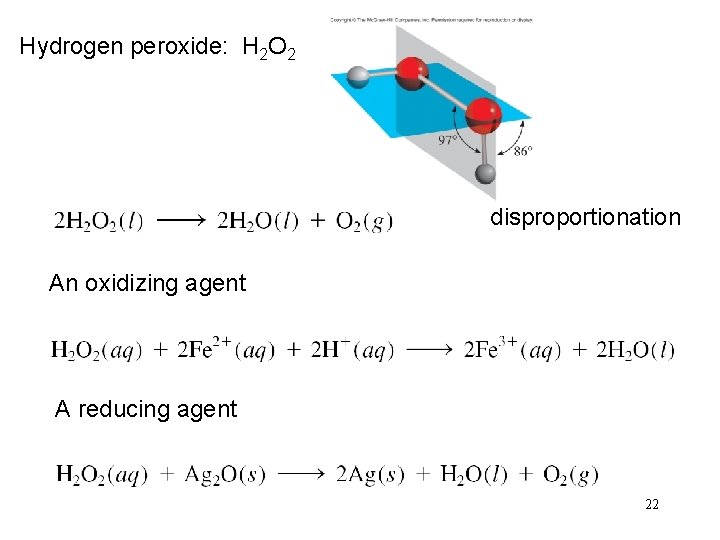
Hydrogen peroxide: H 2 O 2 disproportionation An oxidizing agent A reducing agent 22

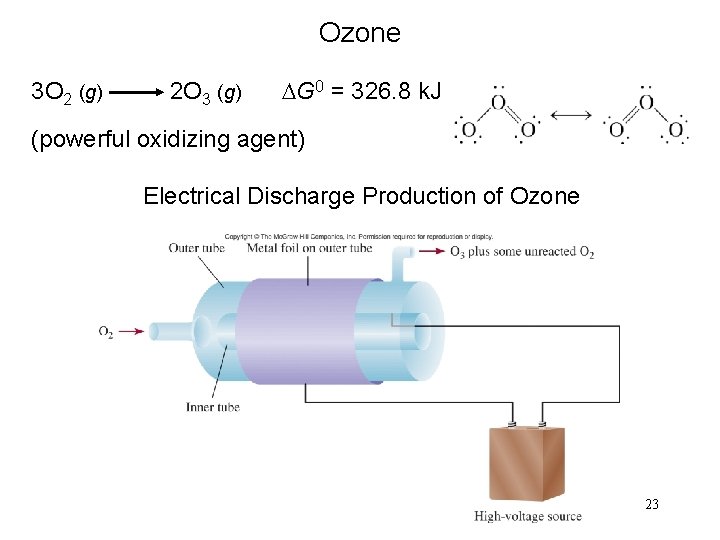
Ozone 3 O 2 (g) 2 O 3 (g) DG 0 = 326. 8 k. J (powerful oxidizing agent) Electrical Discharge Production of Ozone 23

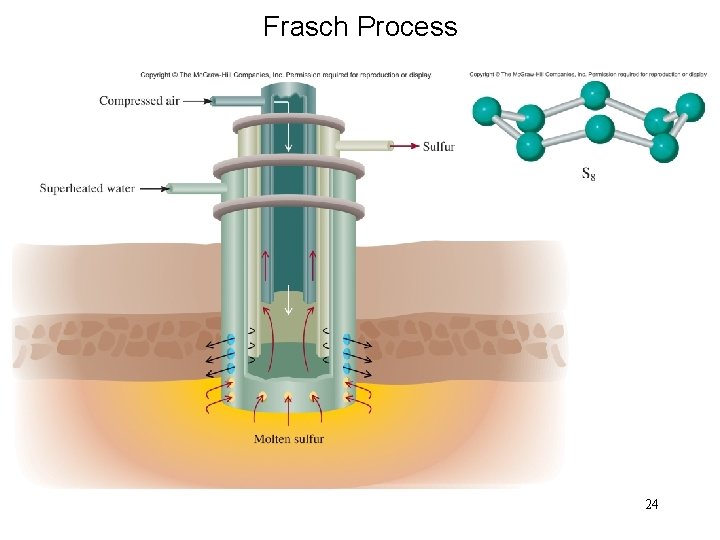
Frasch Process 24

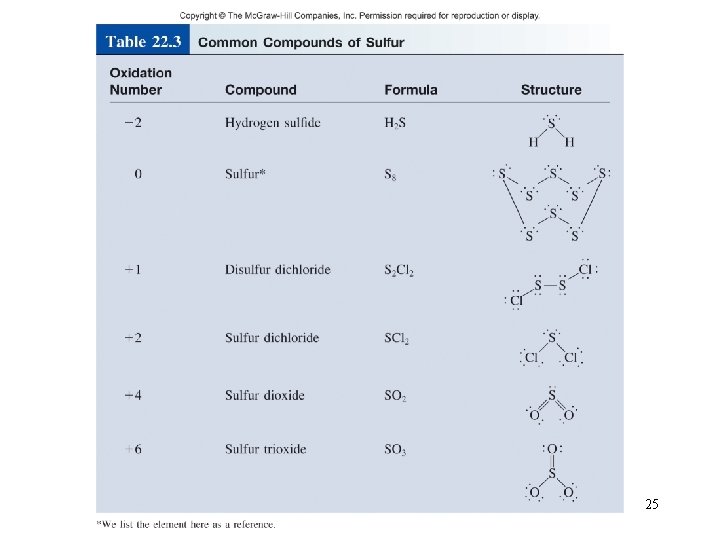
25

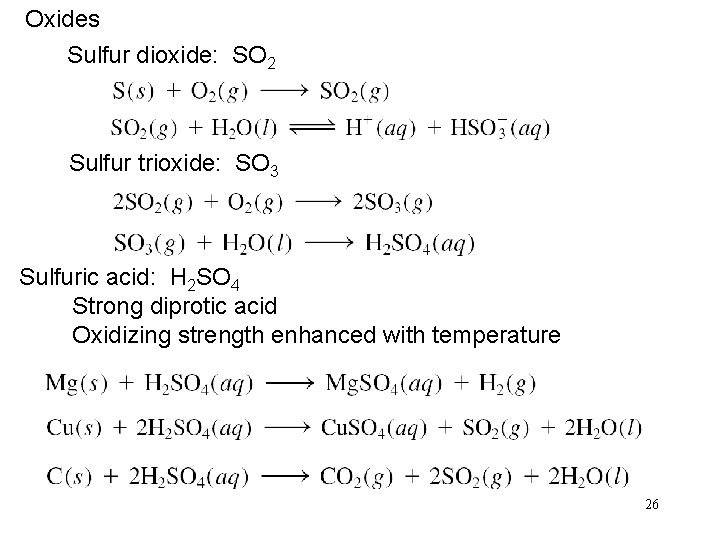
Oxides Sulfur dioxide: SO 2 Sulfur trioxide: SO 3 Sulfuric acid: H 2 SO 4 Strong diprotic acid Oxidizing strength enhanced with temperature 26

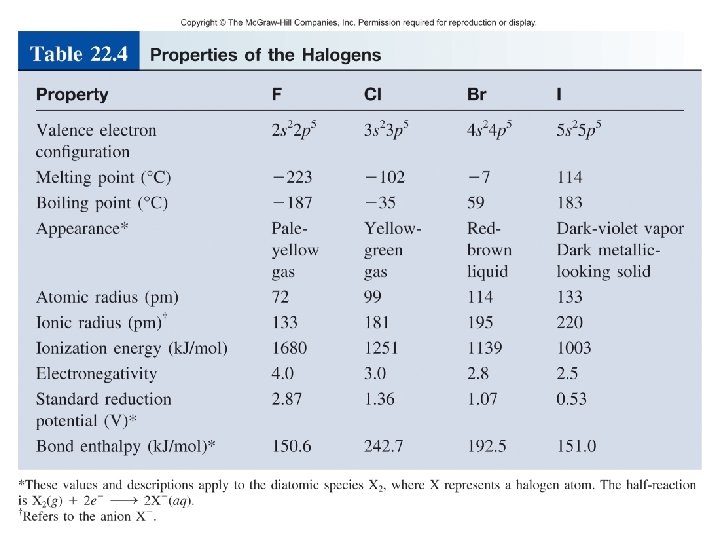
27

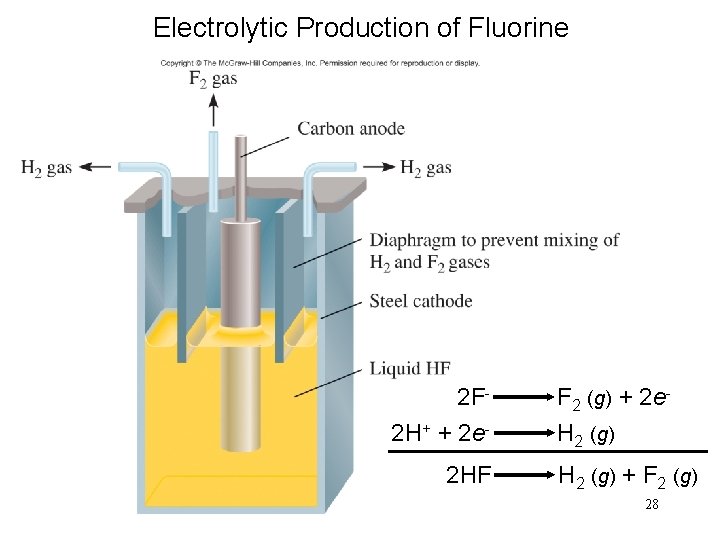
Electrolytic Production of Fluorine 2 F- F 2 (g) + 2 e 2 H+ + 2 e- H 2 (g) 2 HF H 2 (g) + F 2 (g) 28

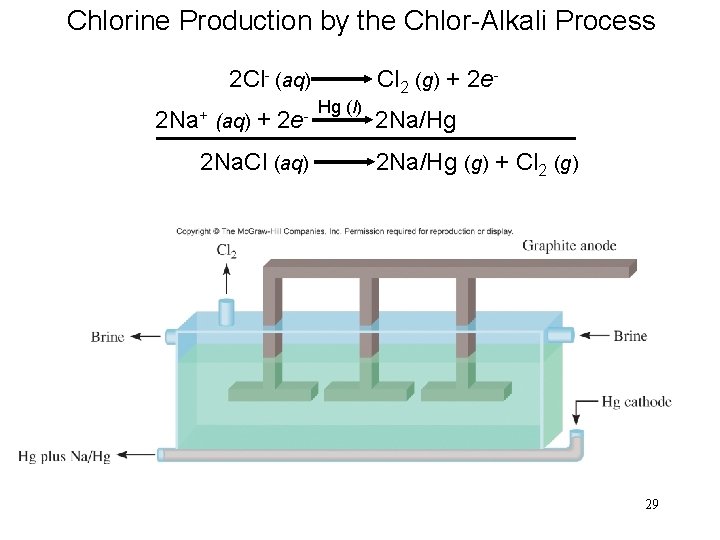
Chlorine Production by the Chlor-Alkali Process 2 Cl- (aq) Cl 2 (g) + 2 e. Hg (l) 2 Na+ (aq) + 2 e- 2 Na/Hg 2 Na. Cl (aq) 2 Na/Hg (g) + Cl 2 (g) 29

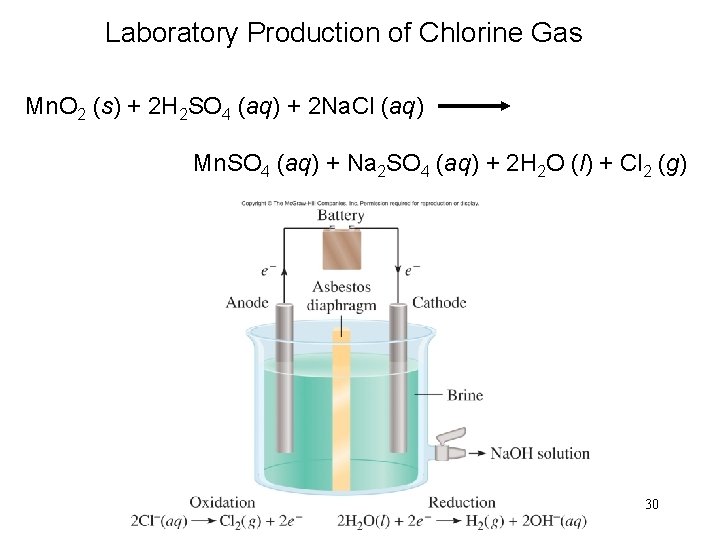
Laboratory Production of Chlorine Gas Mn. O 2 (s) + 2 H 2 SO 4 (aq) + 2 Na. Cl (aq) Mn. SO 4 (aq) + Na 2 SO 4 (aq) + 2 H 2 O (l) + Cl 2 (g) 30

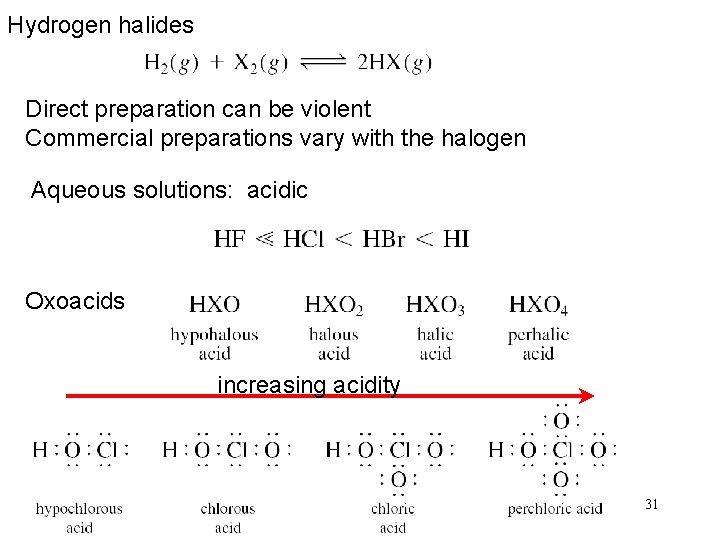
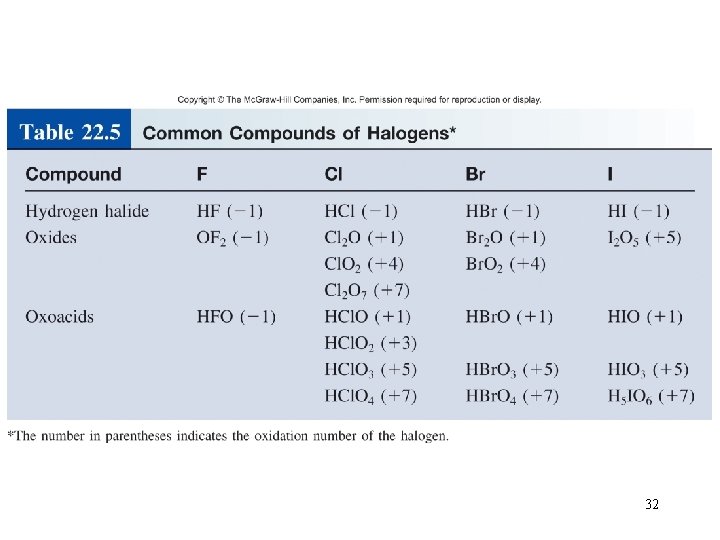
Hydrogen halides Direct preparation can be violent Commercial preparations vary with the halogen Aqueous solutions: acidic Oxoacids increasing acidity 31

32