Elements Compounds Mixtures Comparing Elements Compounds Mixtures Elements







- Slides: 15

Elements, Compounds & Mixtures

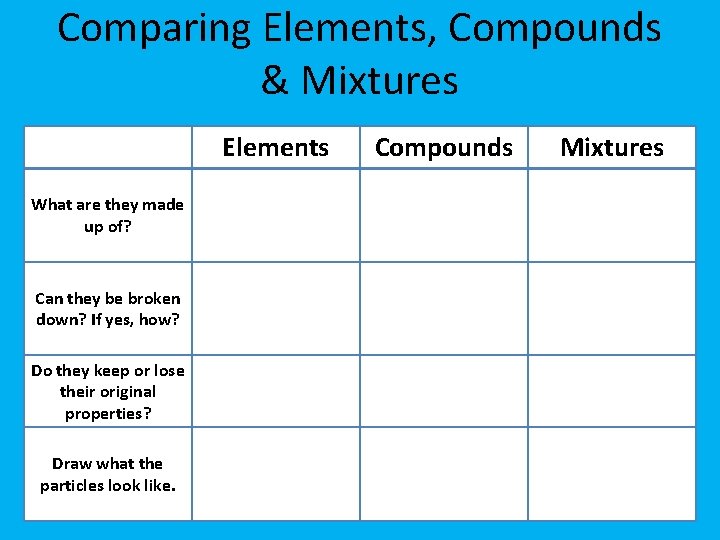
Comparing Elements, Compounds & Mixtures Elements What are they made up of? Can they be broken down? If yes, how? Do they keep or lose their original properties? Draw what the particles look like. Compounds Mixtures

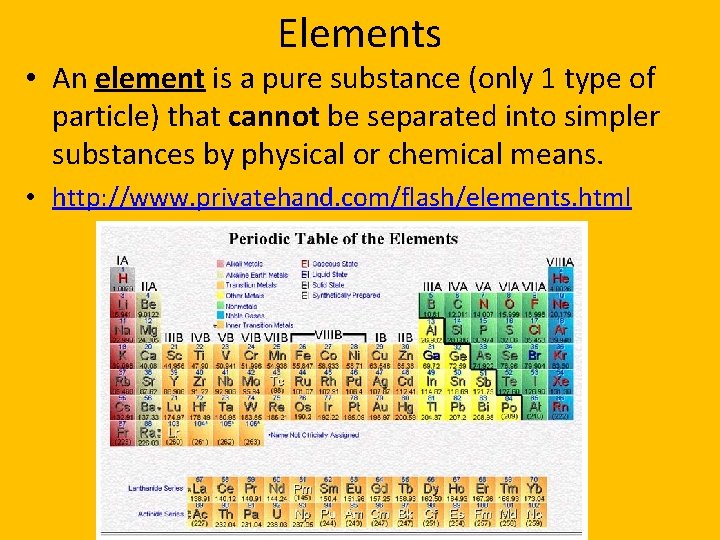
Elements • An element is a pure substance (only 1 type of particle) that cannot be separated into simpler substances by physical or chemical means. • http: //www. privatehand. com/flash/elements. html

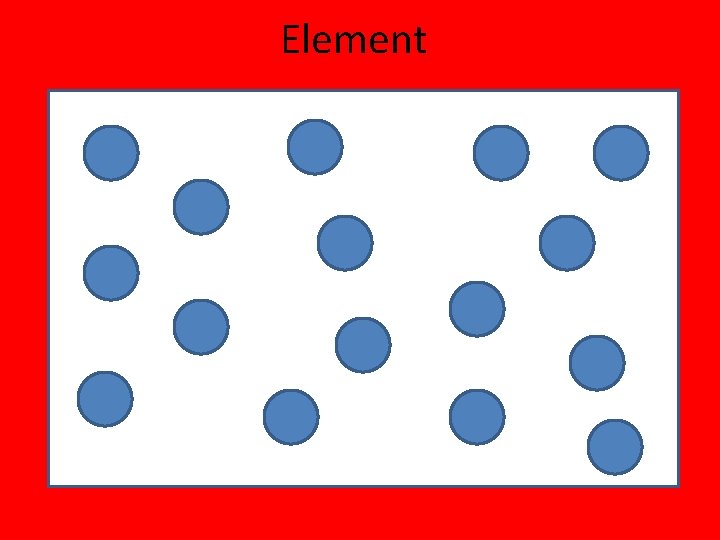
Element

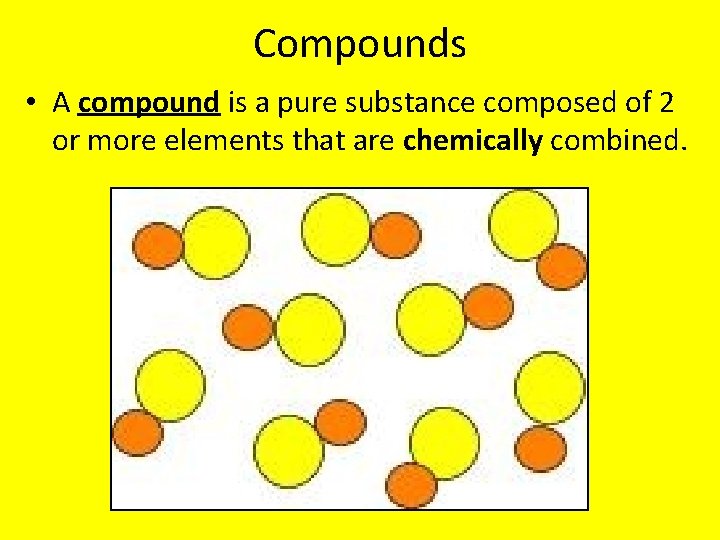
Compounds • A compound is a pure substance composed of 2 or more elements that are chemically combined.

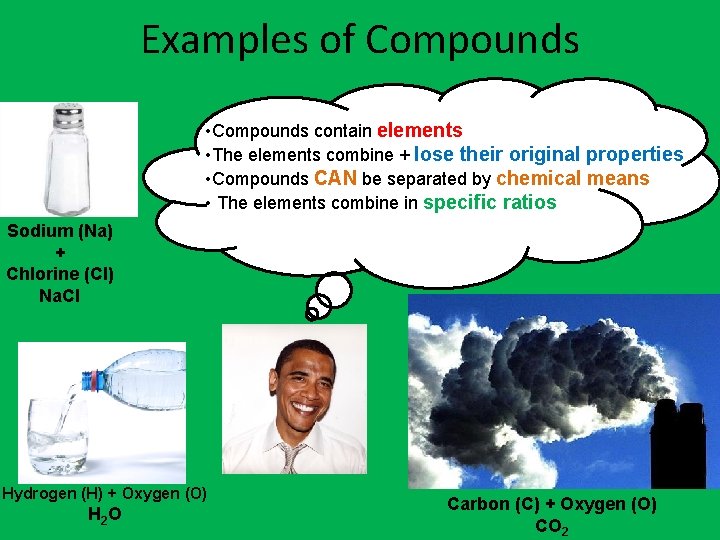
Examples of Compounds • Compounds contain elements • The elements combine + lose their original properties • Compounds CAN be separated by chemical means • The elements combine in specific ratios Sodium (Na) + Chlorine (Cl) Na. Cl Hydrogen (H) + Oxygen (O) H 2 O Carbon (C) + Oxygen (O) CO 2

Mixtures • A mixture is a combination of 2 or more substances that are not chemically combined.

Examples of Mixtures chicken noodle soup salad pizza – yes, that would be Yoda… Properties of Mixtures: • Each substance in a mixture keeps its identity • You can physically separate them • They also contain elements, compounds, or both • And they can be formed using any ratio of components

Mixtures may be homogeneous or heterogeneous • The prefix “homo” indicates the same • Homogeneous mixtures have the same appearance and properties throughout the mixture • The prefix “hetero” indicates difference • Heterogeneous mixtures consist of visibly different substances

Types of Mixtures • There are THREE types of mixtures: –Solutions –Suspensions –Colloids

Solutions • A solution is a mixture that appears to be a single substance, but it is actually composed of 2 or more substances that are distributed evenly amongst each other. – SOLUTIONS ARE HOMOGENEOUS carbon dioxide is dissolved in water sugar & Kool-Aid powder are dissolved in water salt is dissolved in water

Suspensions • A suspension is a mixture in which particles of a material are dispersed throughout a liquid or gas, but are large enough that they settle out. – SUSPENSIONS ARE HETEROGENEOUS That water is disgusting! muddy water Italian dressing

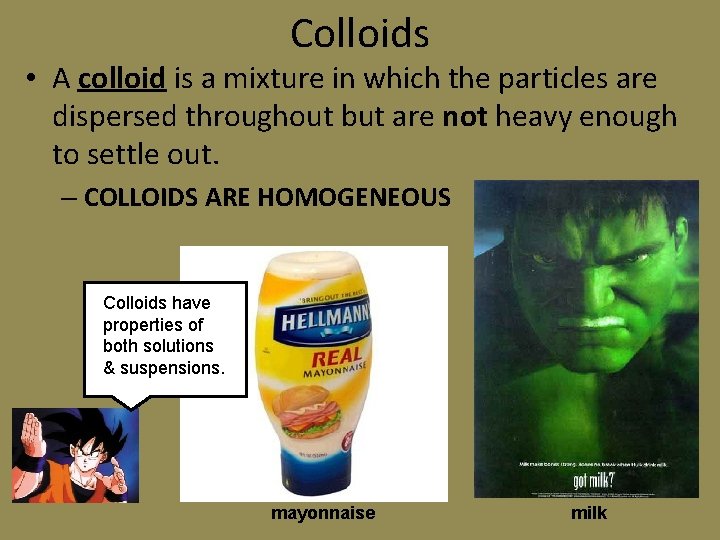
Colloids • A colloid is a mixture in which the particles are dispersed throughout but are not heavy enough to settle out. – COLLOIDS ARE HOMOGENEOUS Colloids have properties of both solutions & suspensions. mayonnaise milk

Comparing Elements, Compounds & Mixtures Elements What are they made up of? Can they be broken down? If yes, how? Do they keep or lose their original properties? Draw what the particles look like. Compounds Mixtures

Identify the following with as many terms as apply 1. 2. 3. 4. 5. 6. 7. 8. Table salt Salad Mayonnaise Italian dressing Pepsi Oxygen Hydrogen Water Mixture Element Compound Suspension Colloid Solution Homogeneous Heterogeneous