Chapter 4 Elements Compounds and Mixtures Oh My























- Slides: 34

Chapter 4 Elements, Compounds, and Mixtures … Oh My!

Section 1 – Elements A lot of this Aabe will review

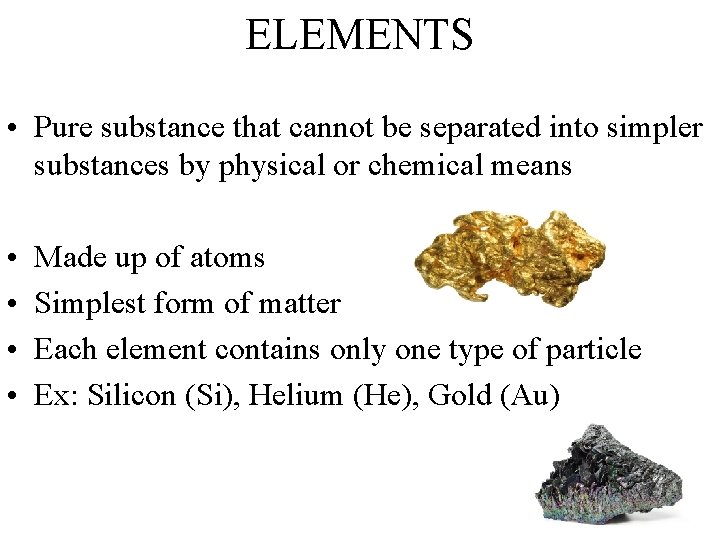
ELEMENTS • Pure substance that cannot be separated into simpler substances by physical or chemical means • • Made up of atoms Simplest form of matter Each element contains only one type of particle Ex: Silicon (Si), Helium (He), Gold (Au)

PROPERTIES • Each element can be identified by its unique set of characteristic properties. • Physical Properties: boiling point, melting point, density, • Chemical Properties: reactivity with acid, flammability

• Classifying Elements. – Grouped into categories by the properties they share. – Elements in same group have shared properties.


3 CATEGORIES

3 CATEGORIES

3 CATEGORIES

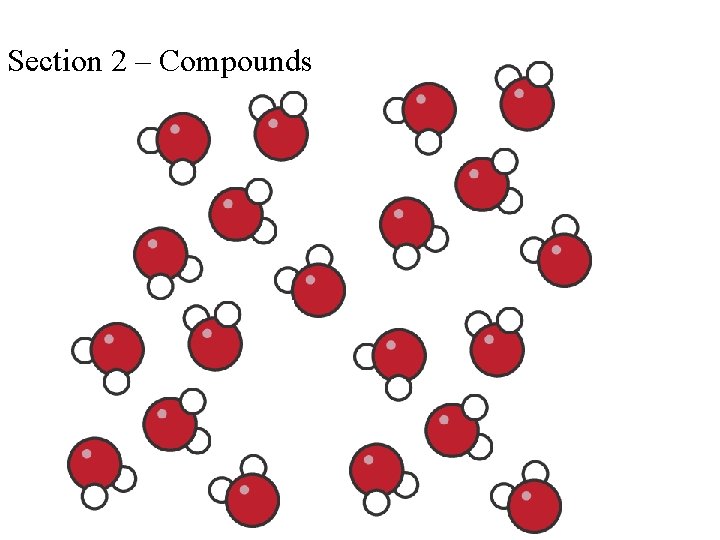
Section 2 – Compounds

COMPOUNDS • A pure substance composed of two or more elements that are chemically combined. • Elements combine by reacting, or undergoing a chemical change, with one another

A particle of a compound is a molecule Two or more atoms join together to form molecules


PROPERTIES • Each compound has a unique set of properties just like elements. • Compounds have different properties than the elements of which they are made.

Sodium Chloride (Na. Cl) – table salt Sodium is a soft, silvery white metal that reacts violently with water Chlorine is a poisonous, greenish yellow gas Sodium chloride is a white solid. It dissolves easily in water and is safe to eat

• Compounds can be broken down into elements or simpler compounds through chemical changes. • Energy can be added to break down a compound: Ø heat Ø electrical current

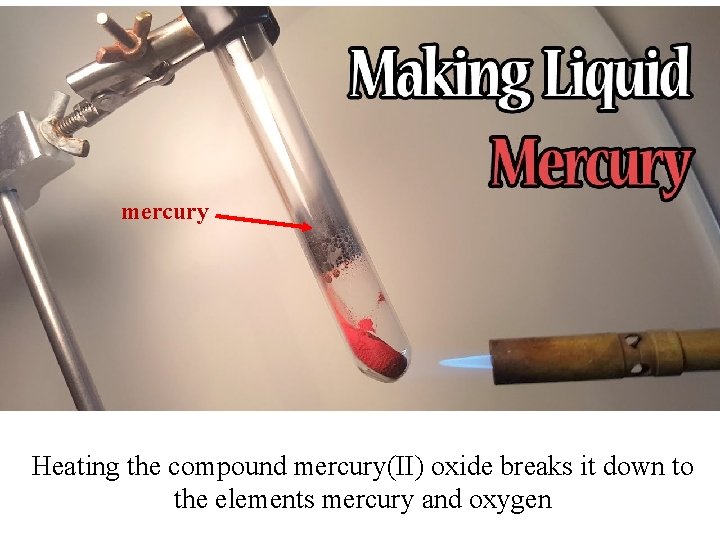
mercury Heating the compound mercury(II) oxide breaks it down to the elements mercury and oxygen



Section 3 – Mixtures

SEPARATING MIXTURES • By physical change – physically separating • Other examples: sorting, filtering, heating, cooling, distilling.

MIXTURES Made of two or more substances – Any combination of elements, compounds, or both Properties of Mixtures • NOT chemically combined – No chemical change occurs – No change to substances identity • Separated by physical means • Formed using any ratio of components (not set like in a compound) • Examples: salt water, steel, air, cereal, pizza, chicken soup, salad

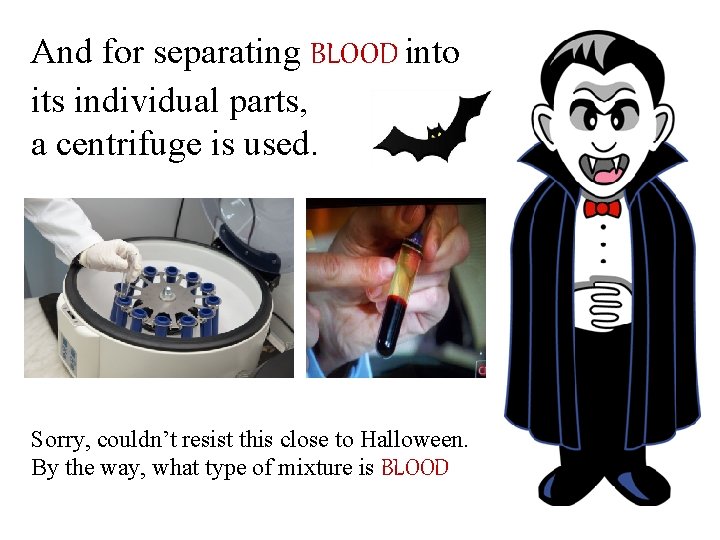
And for separating BLOOD into its individual parts, a centrifuge is used. Sorry, couldn’t resist this close to Halloween. By the way, what type of mixture is BLOOD

Two Kinds of Mixtures 1. homogeneous mixture - uniform composition - small particle evenly distributed 2. heterogeneous mixture - not evenly mixed - particles not uniformly distributed - easily separated physically



SOLUTIONS • A mixture that appears to be a single substance. • It has an even distribution of particles throughout the mixture Homogeneous mixture are solutions.

Solutions are formed by dissolving solute into solvent – Solute – the substance that is dissolving – Solvent – the substance that the solute is dissolving in The solute must be soluble (able to dissolve) in the solvent to make a solution.

PROPERTIES OF SOLUTIONS • Concentration – measure of the amount of solute dissolved in a solvent. – Concentrated – solution has more solute – Dilute – solution has less solute • Saturation – how much solute a solvent can hold – Unsaturated – solution can hold more solute – Saturated – solution has all the solute it can hold

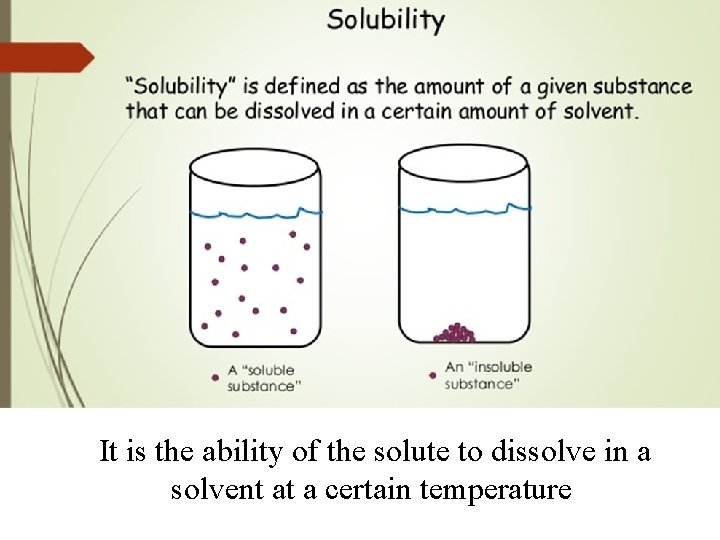
It is the ability of the solute to dissolve in a solvent at a certain temperature

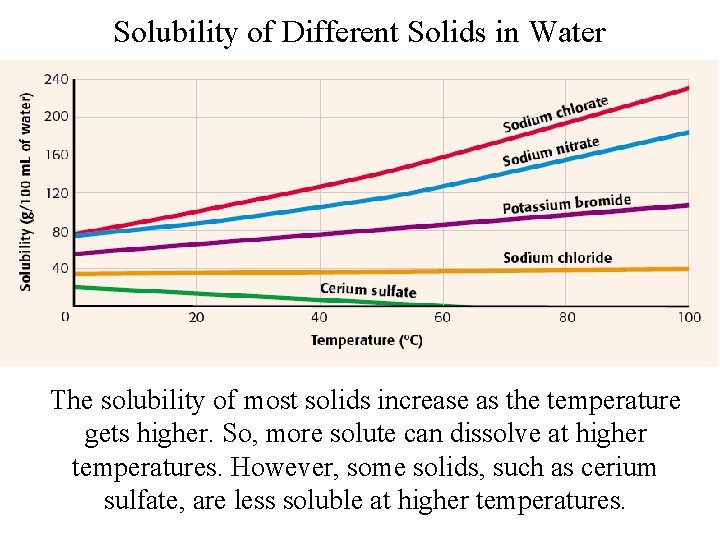
Solubility of Different Solids in Water The solubility of most solids increase as the temperature gets higher. So, more solute can dissolve at higher temperatures. However, some solids, such as cerium sulfate, are less soluble at higher temperatures.

SUSPENSIONS • Particles are large enough to separate themselves from a solvent based on density. – Insoluble – particles not able to dissolve – Heterogeneous mixture – Examples: chicken soup, salad dressing.

COLLOIDS • Particles are small enough to mix with a solvent but not large enough to separate out. • Heterogeneous mixture • Properties of both solutions and suspensions – Spreads out like a solution – Does not dissolve like a suspension • Examples: JELL-O, fog, mayonnaise
