Chemistry 100 Chapter 5 Molecules Compounds Elements Compounds





- Slides: 29

Chemistry 100 Chapter 5 Molecules & Compounds

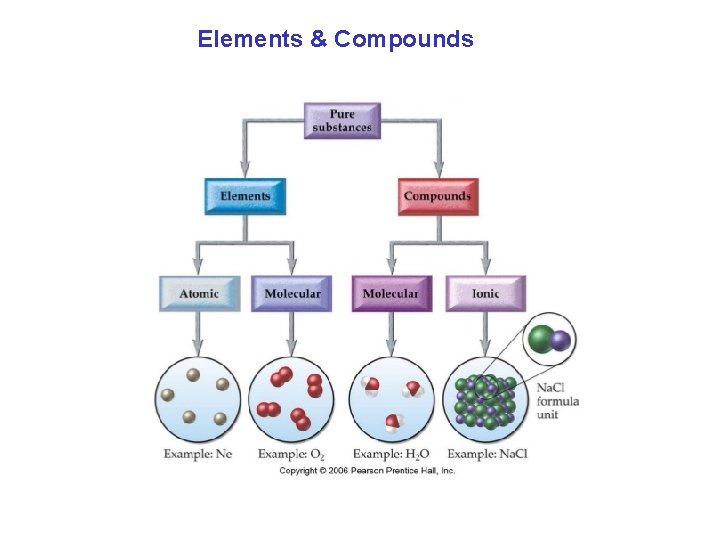
Elements & Compounds

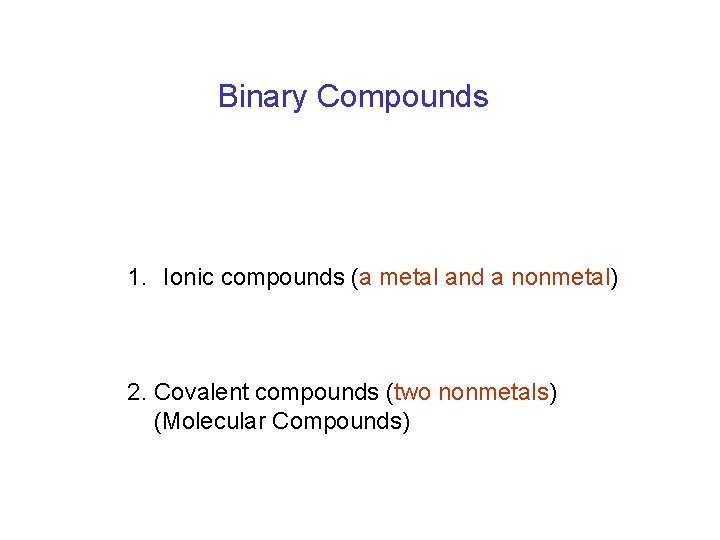
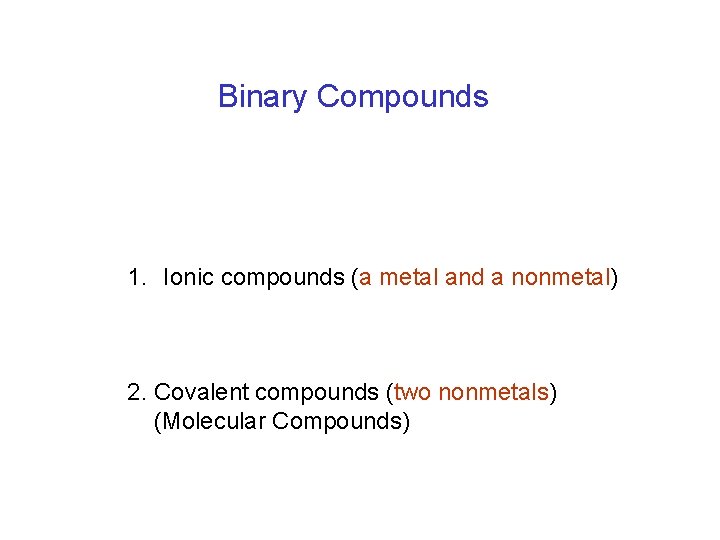
Binary Compounds 1. Ionic compounds (a metal and a nonmetal) 2. Covalent compounds (two nonmetals) (Molecular Compounds)

Binary Compounds 1. Ionic compounds (a metal and a nonmetal)

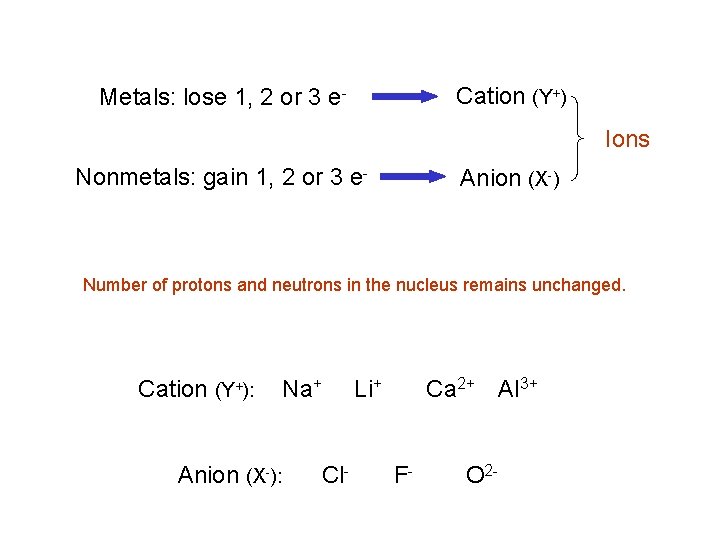
Cation (Y+) Metals: lose 1, 2 or 3 e- Ions Nonmetals: gain 1, 2 or 3 e- Anion (X-) Number of protons and neutrons in the nucleus remains unchanged. Cation (Y+): Anion (X-): Na+ Cl- Li+ Ca 2+ Al 3+ F- O 2 -

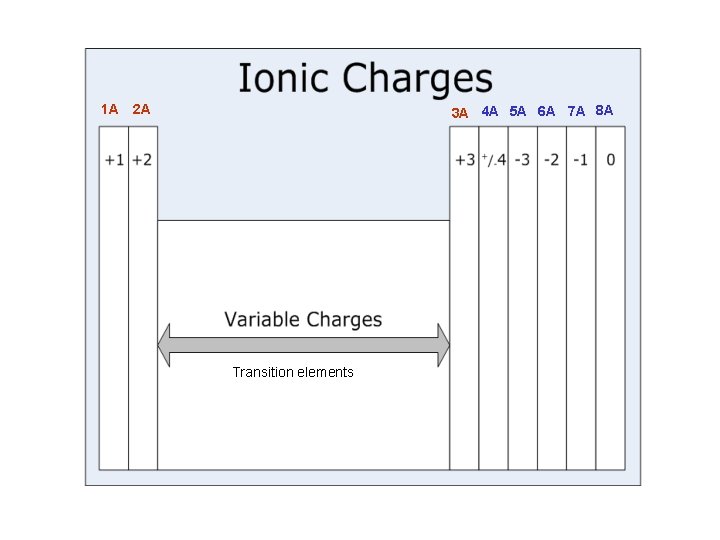
1 A 2 A 3 A 4 A 5 A 6 A 7 A 8 A Transition elements

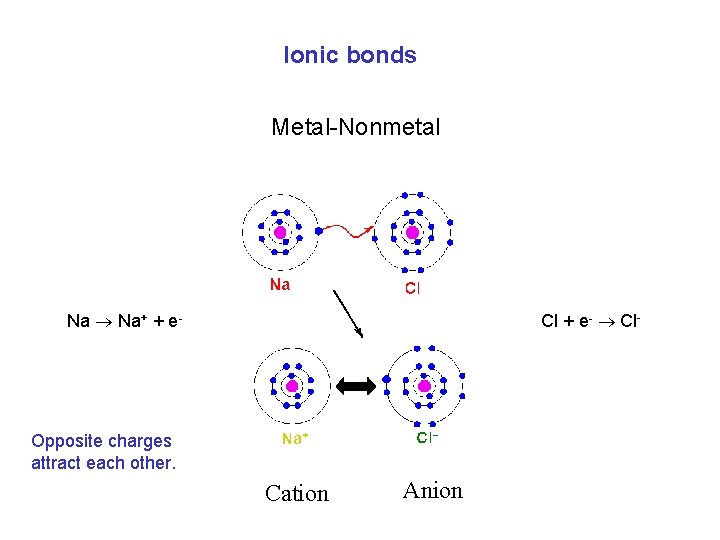
Ionic bonds Metal-Nonmetal Na Na+ + e- Cl + e- Cl- Opposite charges attract each other. Cation Anion

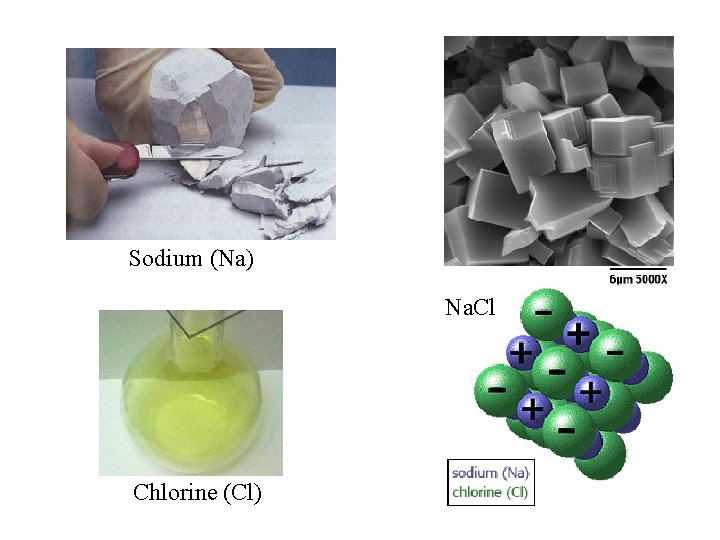
Sodium (Na) Na. Cl Chlorine (Cl)

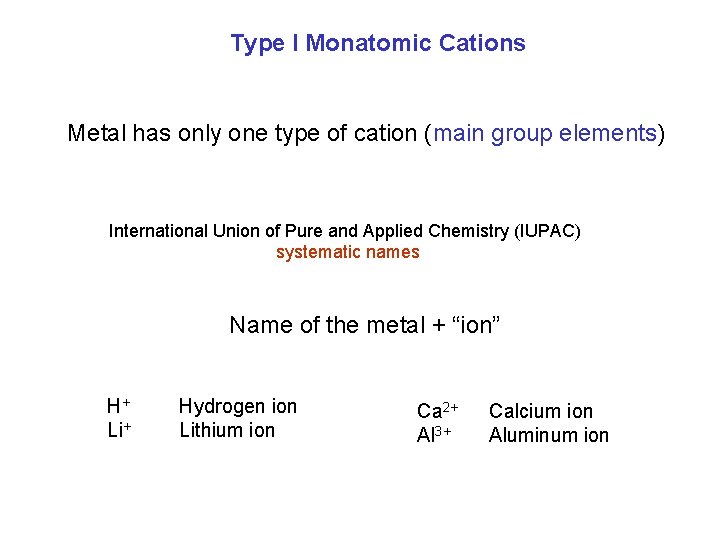
Type I Monatomic Cations Metal has only one type of cation (main group elements) International Union of Pure and Applied Chemistry (IUPAC) systematic names Name of the metal + “ion” H+ Li+ Hydrogen ion Lithium ion Ca 2+ Al 3+ Calcium ion Aluminum ion

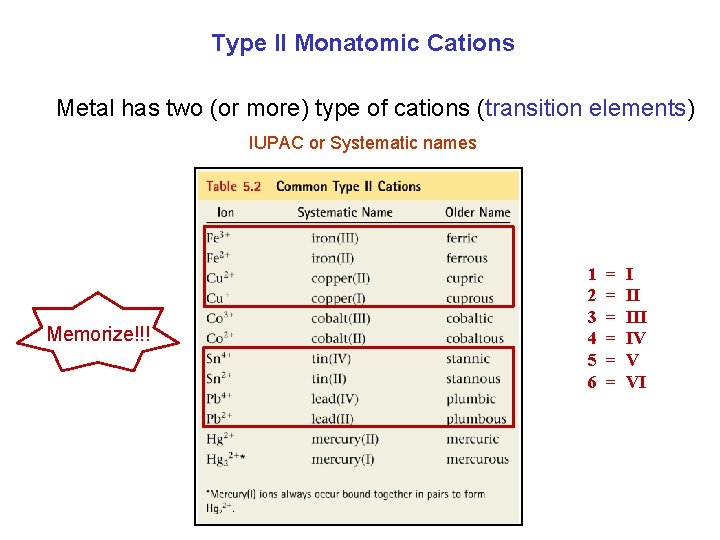
Type II Monatomic Cations Metal has two (or more) type of cations (transition elements) IUPAC or Systematic names Memorize!!! 1 2 3 4 5 6 = = = I II IV V VI

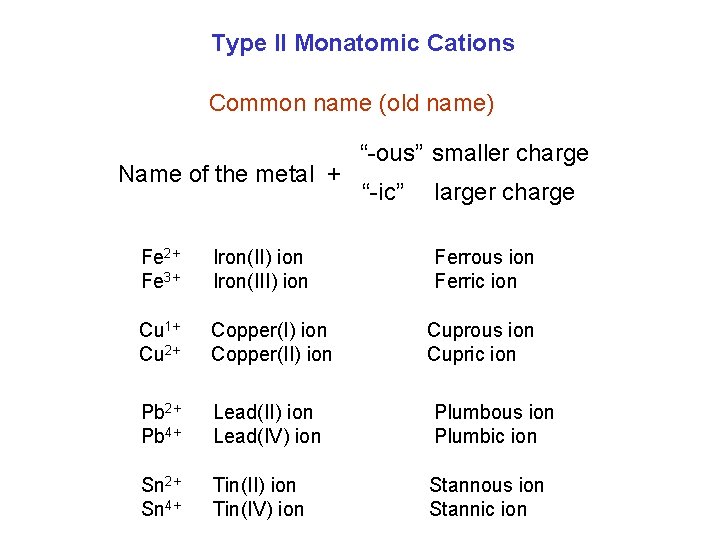
Type II Monatomic Cations Common name (old name) Name of the metal + “-ous” smaller charge “-ic” larger charge Fe 2+ Fe 3+ Iron(II) ion Iron(III) ion Ferrous ion Ferric ion Cu 1+ Cu 2+ Copper(I) ion Copper(II) ion Pb 2+ Pb 4+ Lead(II) ion Lead(IV) ion Plumbous ion Plumbic ion Sn 2+ Sn 4+ Tin(II) ion Tin(IV) ion Stannous ion Stannic ion Cuprous ion Cupric ion

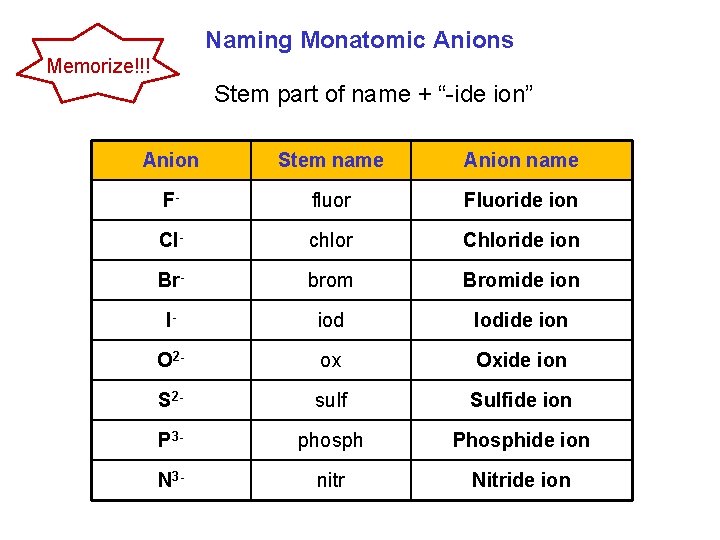
Naming Monatomic Anions Memorize!!! Stem part of name + “-ide ion” Anion Stem name Anion name F- fluor Fluoride ion Cl- chlor Chloride ion Br- brom Bromide ion I- iod Iodide ion O 2 - ox Oxide ion S 2 - sulf Sulfide ion P 3 - phosph Phosphide ion N 3 - nitr Nitride ion

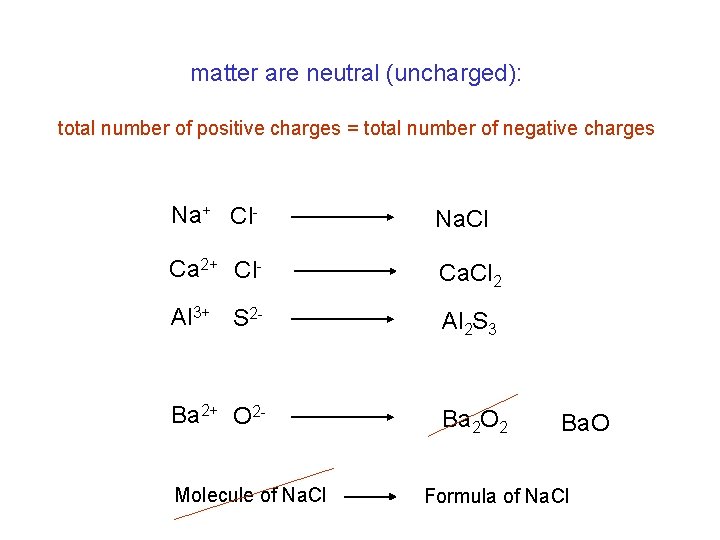
matter are neutral (uncharged): total number of positive charges = total number of negative charges Na+ Cl- Na. Cl Ca 2+ Cl- Ca. Cl 2 Al 3+ S 2 - Al 2 S 3 Ba 2+ O 2 - Ba 2 O 2 Molecule of Na. Cl Ba. O Formula of Na. Cl

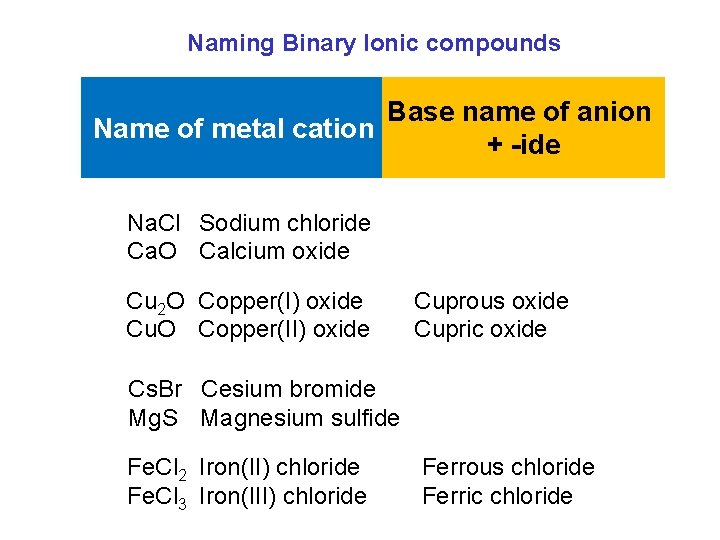
Naming Binary Ionic compounds Base name of anion Name of metal cation + -ide Na. Cl Sodium chloride Ca. O Calcium oxide Cu 2 O Copper(I) oxide Cu. O Copper(II) oxide Cuprous oxide Cupric oxide Cs. Br Cesium bromide Mg. S Magnesium sulfide Fe. Cl 2 Iron(II) chloride Fe. Cl 3 Iron(III) chloride Ferrous chloride Ferric chloride

Binary Compounds 1. Ionic compounds (a metal and a nonmetal) 2. Covalent compounds (two nonmetals) (Molecular Compounds)

Binary Compounds 2. Covalent compounds (two nonmetals)

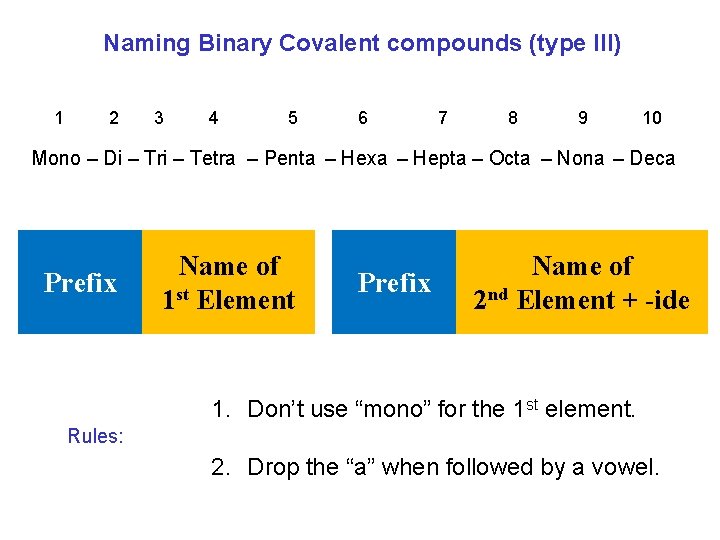
Naming Binary Covalent compounds (type III) 1 2 3 4 5 6 7 8 9 10 Mono – Di – Tri – Tetra – Penta – Hexa – Hepta – Octa – Nona – Deca Prefix Name of 1 st Element Prefix Name of 2 nd Element + -ide 1. Don’t use “mono” for the 1 st element. Rules: 2. Drop the “a” when followed by a vowel.

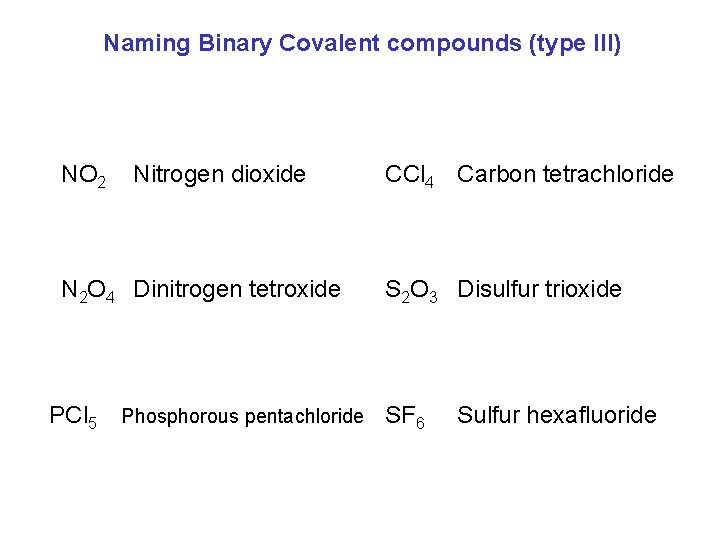
Naming Binary Covalent compounds (type III) NO 2 Nitrogen dioxide N 2 O 4 Dinitrogen tetroxide PCl 5 Phosphorous pentachloride CCl 4 Carbon tetrachloride S 2 O 3 Disulfur trioxide SF 6 Sulfur hexafluoride

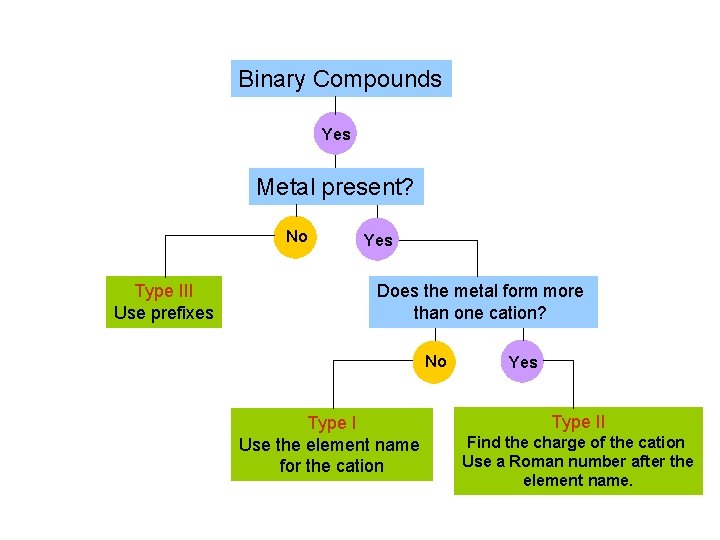
Binary Compounds Yes Metal present? No Type III Use prefixes Yes Does the metal form more than one cation? No Type I Use the element name for the cation Yes Type II Find the charge of the cation Use a Roman number after the element name.

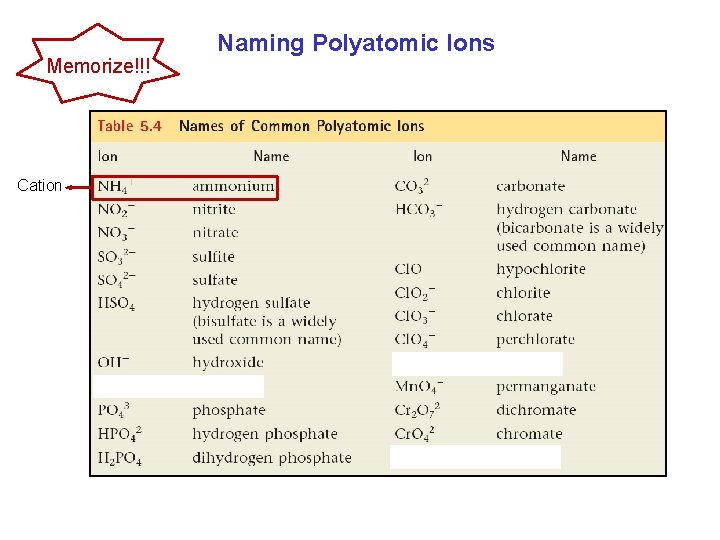
Naming Polyatomic Ionic Compounds They contain more than two elements.

Memorize!!! Cation Naming Polyatomic Ions

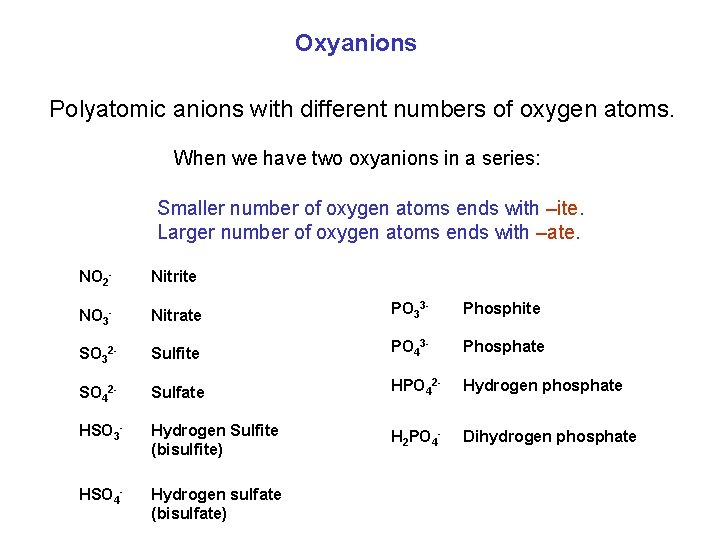
Oxyanions Polyatomic anions with different numbers of oxygen atoms. When we have two oxyanions in a series: Smaller number of oxygen atoms ends with –ite. Larger number of oxygen atoms ends with –ate. NO 2 - Nitrite NO 3 - Nitrate PO 33 - Phosphite SO 32 - Sulfite PO 43 - Phosphate SO 42 - Sulfate HPO 42 - Hydrogen phosphate HSO 3 - Hydrogen Sulfite (bisulfite) H 2 PO 4 - Dihydrogen phosphate HSO 4 - Hydrogen sulfate (bisulfate)

Oxyanions When we have more than two oxyanions in a series: (Fewest oxygen atoms) Prefix hypo- (Most oxygen atoms) Prefix per- Cl. O- hypochlorite Cl. O 2 - chlorite Cl. O 3 - chlorate Cl. O 4 - perchlorate

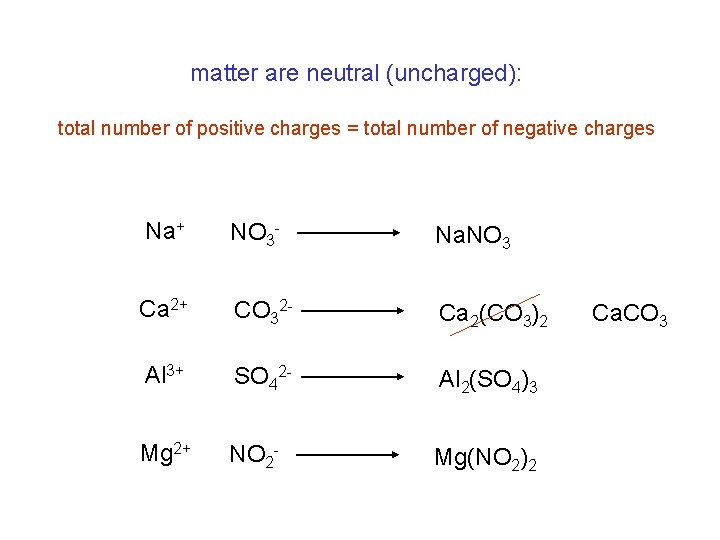
matter are neutral (uncharged): total number of positive charges = total number of negative charges Na+ NO 3 - Na. NO 3 Ca 2+ CO 32 - Ca 2(CO 3)2 Al 3+ SO 42 - Al 2(SO 4)3 Mg 2+ NO 2 - Mg(NO 2)2 Ca. CO 3

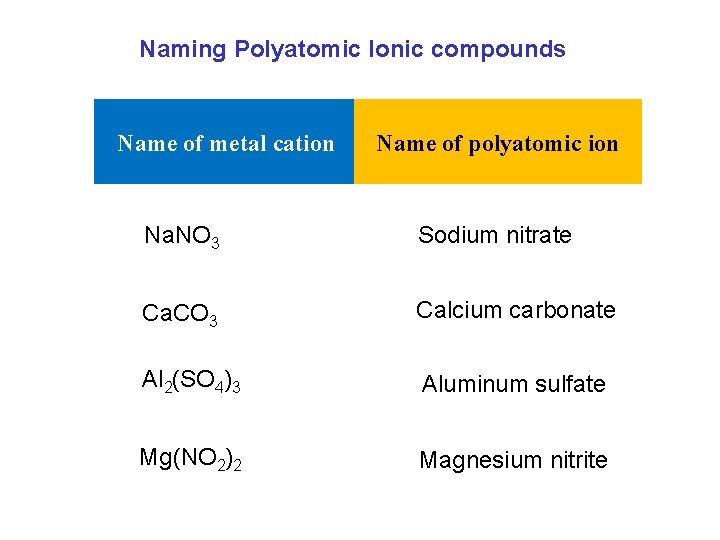
Naming Polyatomic Ionic compounds Name of metal cation Name of polyatomic ion Na. NO 3 Sodium nitrate Ca. CO 3 Calcium carbonate Al 2(SO 4)3 Aluminum sulfate Mg(NO 2)2 Magnesium nitrite

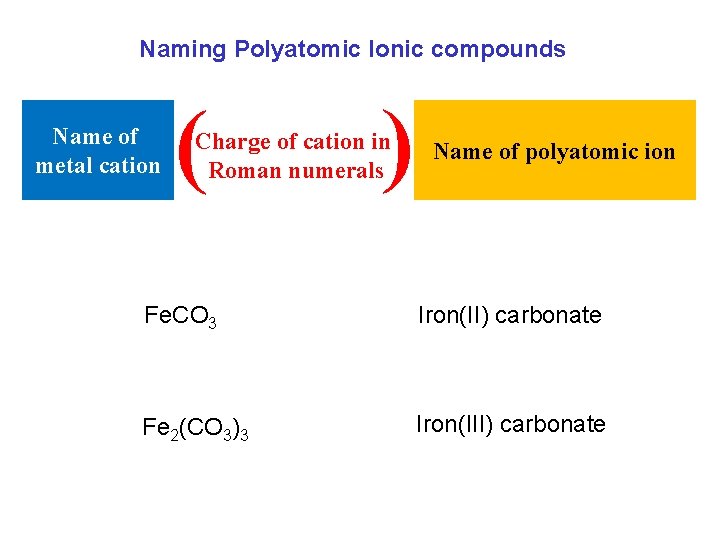
Naming Polyatomic Ionic compounds Name of metal cation ( ) Charge of cation in Roman numerals Name of polyatomic ion Fe. CO 3 Iron(II) carbonate Fe 2(CO 3)3 Iron(III) carbonate

Naming acids Acids: sour They produce H+ (proton) in water.

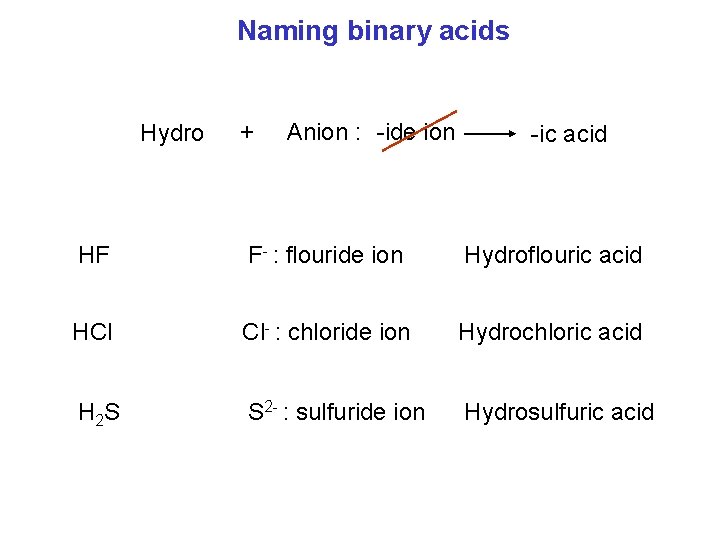
Naming binary acids Hydro + Anion : -ide ion -ic acid HF F- : flouride ion Hydroflouric acid HCl Cl- : chloride ion Hydrochloric acid H 2 S S 2 - : sulfuride ion Hydrosulfuric acid

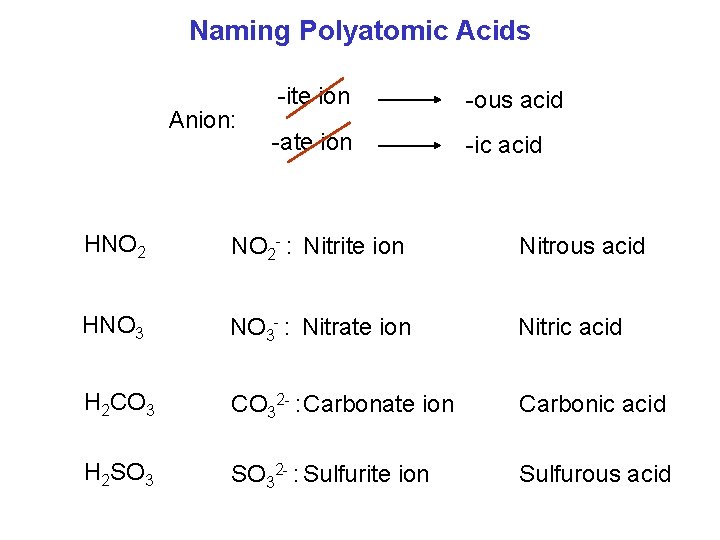
Naming Polyatomic Acids Anion: -ite ion -ous acid -ate ion -ic acid HNO 2 - : Nitrite ion Nitrous acid HNO 3 - : Nitrate ion Nitric acid H 2 CO 32 - : Carbonate ion Carbonic acid H 2 SO 32 - : Sulfurite ion Sulfurous acid