Atoms Elements Compounds and Mixtures Quiz Draw and






- Slides: 11

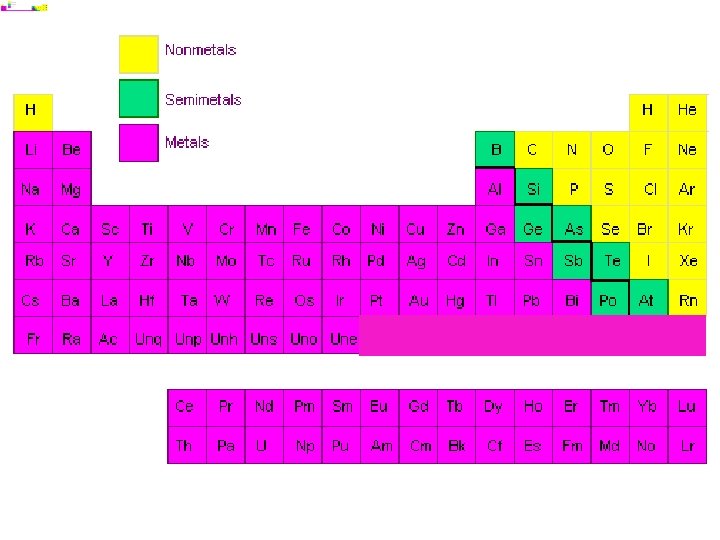
Atoms, Elements, Compounds, and Mixtures Quiz • Draw and name an example of each of the following: – Atom – Element – Compound – Mixture • Explain briefly what each of them are (definition) in your own words.


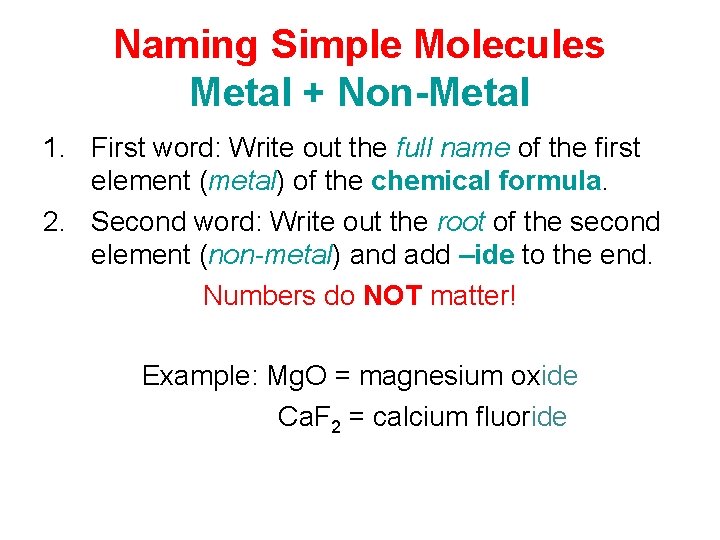
Naming Simple Molecules Metal + Non-Metal 1. First word: Write out the full name of the first element (metal) of the chemical formula. 2. Second word: Write out the root of the second element (non-metal) and add –ide to the end. Numbers do NOT matter! Example: Mg. O = magnesium oxide Ca. F 2 = calcium fluoride

Name that Compound! Chemical Formula • Na. Cl • Cu. O • KBr • Ca. Cl 2 • Zn. S Name 1. Sodium Chloride 2. Copper Oxide 3. Potassium Bromide 4. Calcium Chloride 5. Zinc Sulphide

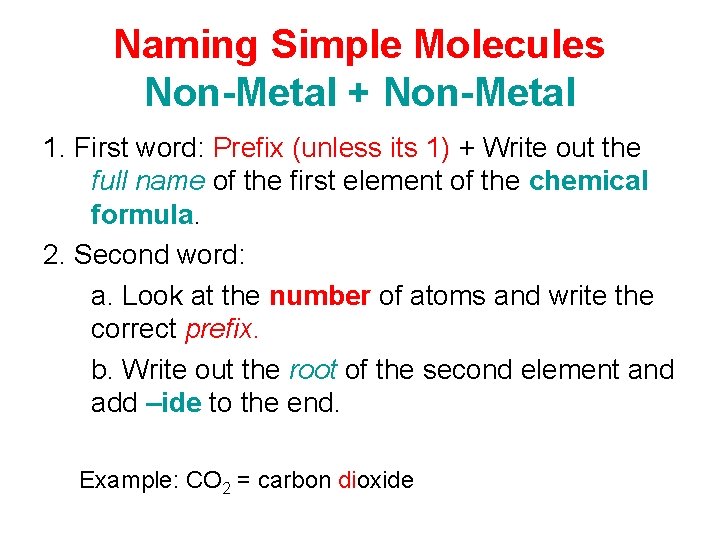
Naming Simple Molecules Non-Metal + Non-Metal 1. First word: Prefix (unless its 1) + Write out the full name of the first element of the chemical formula. 2. Second word: a. Look at the number of atoms and write the correct prefix. b. Write out the root of the second element and add –ide to the end. Example: CO 2 = carbon dioxide

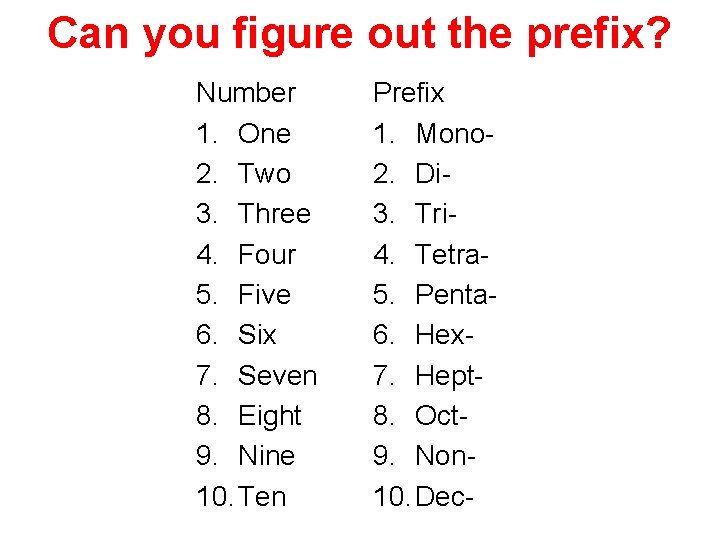
Can you figure out the prefix? Number 1. One 2. Two 3. Three 4. Four 5. Five 6. Six 7. Seven 8. Eight 9. Nine 10. Ten Prefix 1. Mono 2. Di 3. Tri 4. Tetra 5. Penta 6. Hex 7. Hept 8. Oct 9. Non 10. Dec-

Name that Compound! Chemical Formula • NO • SO 2 • CO • CCl 4 Name 1. Nitrogen Monoxide 2. Sulphur Dioxide 3. Carbon Monoxide 4. Carbon Tetrachloride

Name that Compound! Chemical Formula • Mg. CO 3 • Zn. SO 4 • Zn. SO 3 • Na 2 SO 4 • Li. NO 3 • Ca 3(PO 4)2 Name 1. Magnesium Carbonate 2. Zinc Sulphate 3. Zinc Sulphite 4. Sodium Sulphate 5. Lithium Nitrate 6. Calcium Phosphate

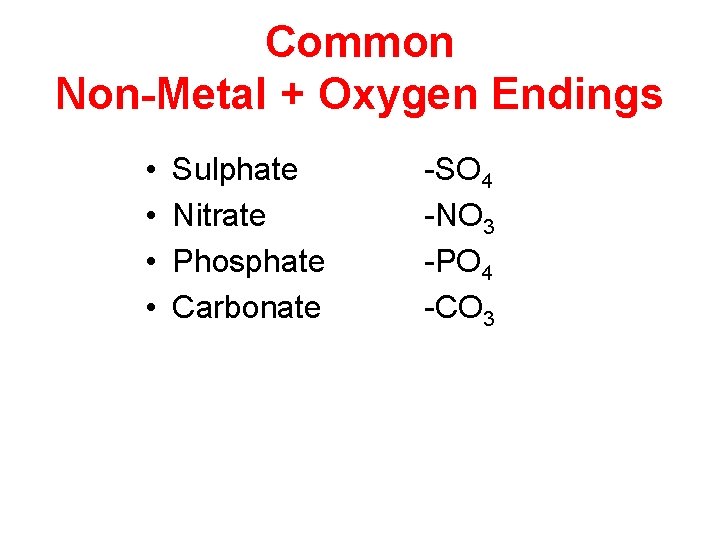
Common Non-Metal + Oxygen Endings • • Sulphate Nitrate Phosphate Carbonate -SO 4 -NO 3 -PO 4 -CO 3

Name the following compounds 1. Li. Cl 2. Ca. F 2 3. KBr 4. Ba. O 5. Mg. CO 3 6. Zn. SO 4 7. Zn. SO 3 8. WCl 3 9. Al. PO 4 10. Na. NO 3 1. Lithium Chloride 2. Calcium Fluoride 3. Potassium Bromide 4. Barium Oxide 5. Magnesium Carbonate 6. Zinc Sulphate 7. Zinc Sulphite 8. Tungsten Chloride 9. Aluminium Phosphate 10. Sodium Nitrate

Name the following compounds a) b) c) d) e) f) g) h) i) j) CO SF 6 SO 2 Si. Cl 4 P 4 O 10 NH 3 PBr 5 PCl 3 Si. O 2 Xe. F 6 1. Carbon Monoxide 2. Sulphur Hexafluoride 3. Sulphur Dioxide 4. Silicon Tetrachloride 5. Tetraphosphorus Decaoxide 6. Nitrogen Trihyride 7. Phosphorous Pentabromide 8. Phosphorous Trichloride 9. Silicon Dioxide 10. Xenon Hexafluoride