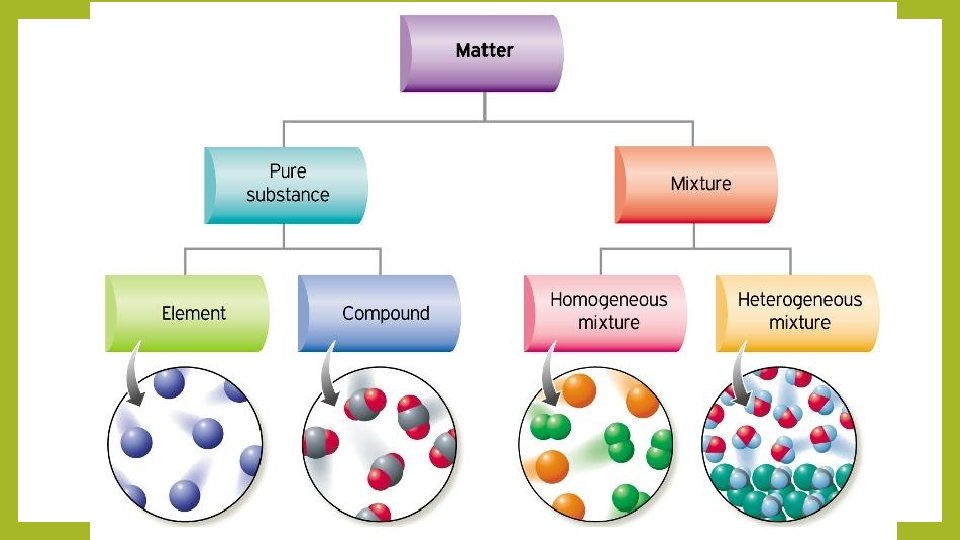
Elements vs Compounds Similarities Elements and compounds are


- Slides: 11

Elements vs Compounds - Similarities • Elements and compounds are both pure substances • This means that they can’t be broken down into simpler substances by physical means

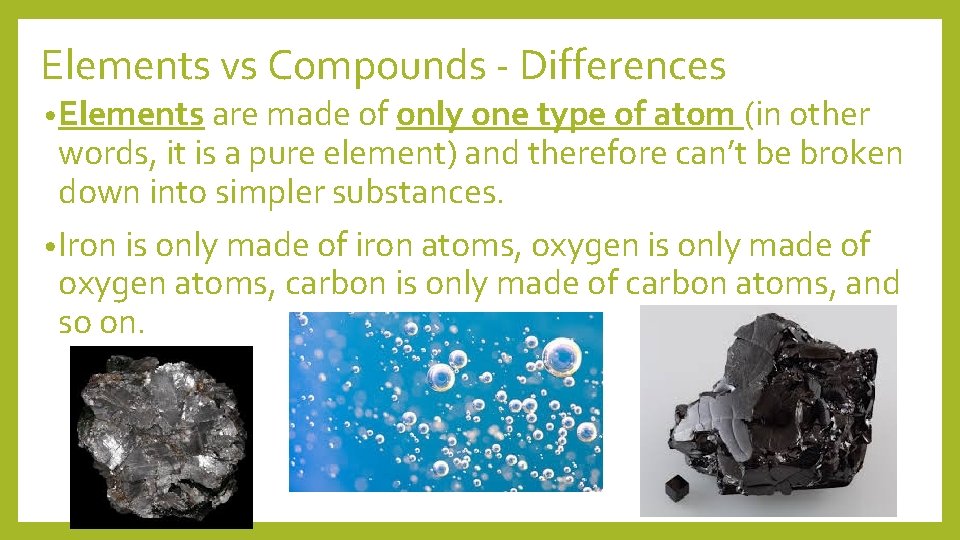
Elements vs Compounds - Differences • Elements are made of only one type of atom (in other words, it is a pure element) and therefore can’t be broken down into simpler substances. • Iron is only made of iron atoms, oxygen is only made of oxygen atoms, carbon is only made of carbon atoms, and so on.

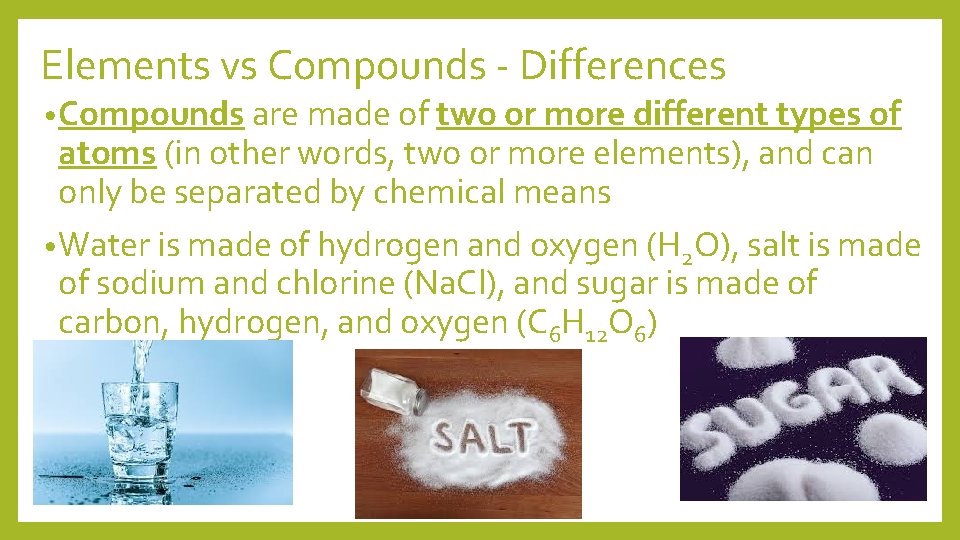
Elements vs Compounds - Differences • Compounds are made of two or more different types of atoms (in other words, two or more elements), and can only be separated by chemical means • Water is made of hydrogen and oxygen (H 2 O), salt is made of sodium and chlorine (Na. Cl), and sugar is made of carbon, hydrogen, and oxygen (C 6 H 12 O 6)

Mixtures • Mixtures are created when different elements and compounds are physically combined and each “ingredient” keeps its own properties. No chemical changes or reactions take place! • Mixtures are NOT pure substances because they can be separated physically. • There are two types of mixtures: heterogeneous and homogeneous

Mixtures • Heterogeneous mixtures are not uniform throughout, so you can see the different parts that make up the mixture • Hetero = different • Homogeneous mixtures are uniform throughout, so you can’t distinguish • Homo = same


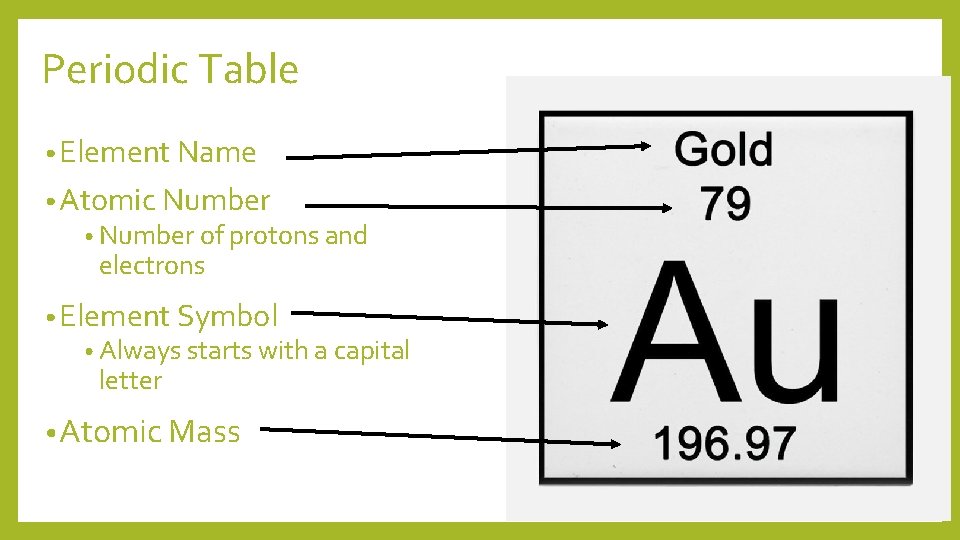
Periodic Table • Element Name • Atomic Number • Number of protons and electrons • Element Symbol • Always starts with a capital letter • Atomic Mass

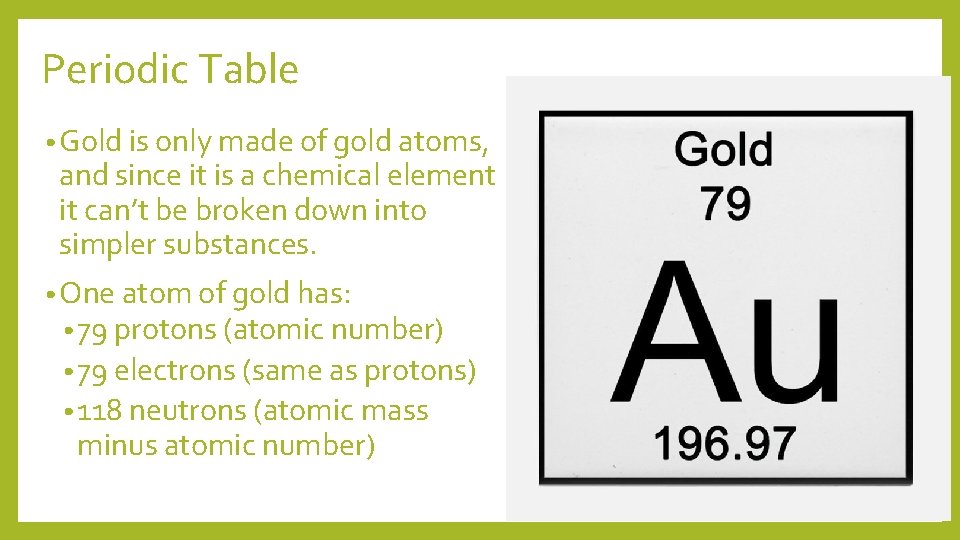
Periodic Table • Gold is only made of gold atoms, and since it is a chemical element it can’t be broken down into simpler substances. • One atom of gold has: • 79 protons (atomic number) • 79 electrons (same as protons) • 118 neutrons (atomic mass minus atomic number)

Chemical Formulas • Chemical formulas are like the “ingredients” of a compound. • Common chemical formulas are H 2 O (water), CO 2 (carbon dioxide), C 6 H 12 O 6 (sugar), Na. Cl (salt), HCl (hydrochloric acid), and C 3 H 8 (propane). • This tells you what elements make up a compound and how many atoms there are of each element.

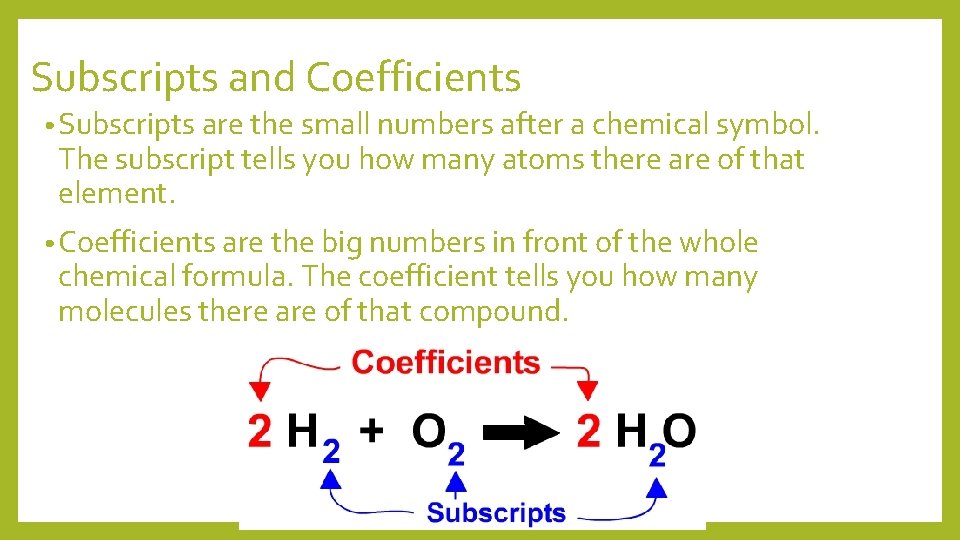
Subscripts and Coefficients • Subscripts are the small numbers after a chemical symbol. The subscript tells you how many atoms there are of that element. • Coefficients are the big numbers in front of the whole chemical formula. The coefficient tells you how many molecules there are of that compound.

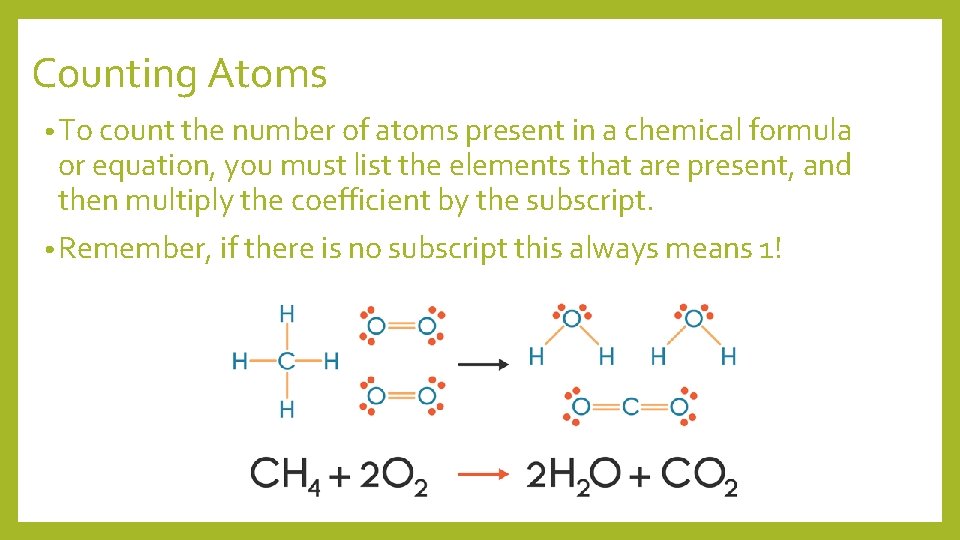
Counting Atoms • To count the number of atoms present in a chemical formula or equation, you must list the elements that are present, and then multiply the coefficient by the subscript. • Remember, if there is no subscript this always means 1!