Atomic Theory Origin The earliest recorded idea about

Atomic Theory

Origin • The earliest recorded idea about the existence of atoms comes from a Greek philosopher named Democritus @400 BC • He theorized that if you were to cut an object in half, then cut it in half again and again over and over… • Eventually you would be left with something too small to cut any more. • This would represent the smallest individual unit of matter. • He gave it the name, “ATOMOS, ” which means UNCUTTABLE.

Atomic Theory An Englishman named John Dalton is credited with developing the first modern ideas about atoms in 1808: – All matter is composed of atoms. – The “building blocks of the universe” – Atoms of each element are the same size, mass, etc. , but are different from atoms of other elements. – Each element has a unique type of atom – Atoms can’t be subdivided (cut) or destroyed. – Atoms are the smallest individual unit of matter – Has since been falsified by evidence (nuclear reactions)
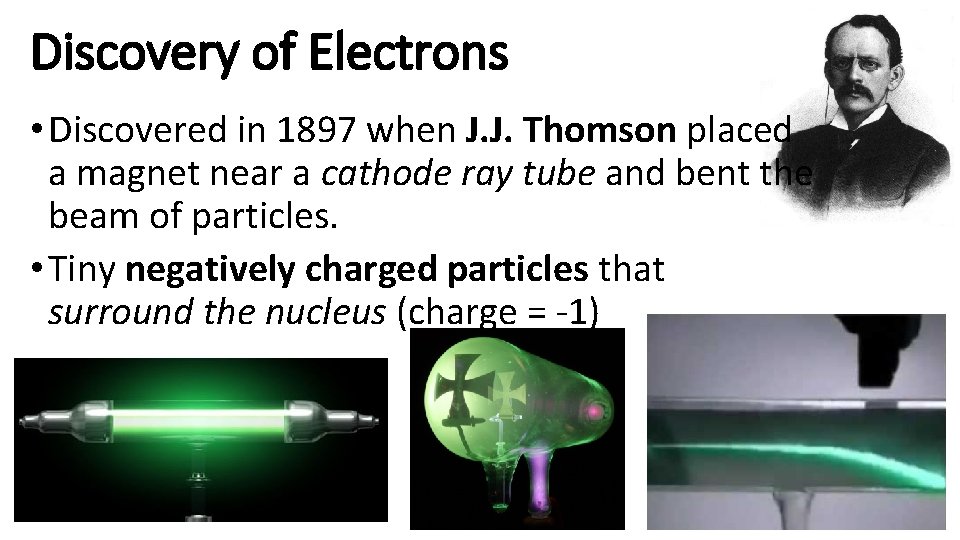
Discovery of Electrons • Discovered in 1897 when J. J. Thomson placed a magnet near a cathode ray tube and bent the beam of particles. • Tiny negatively charged particles that surround the nucleus (charge = -1)
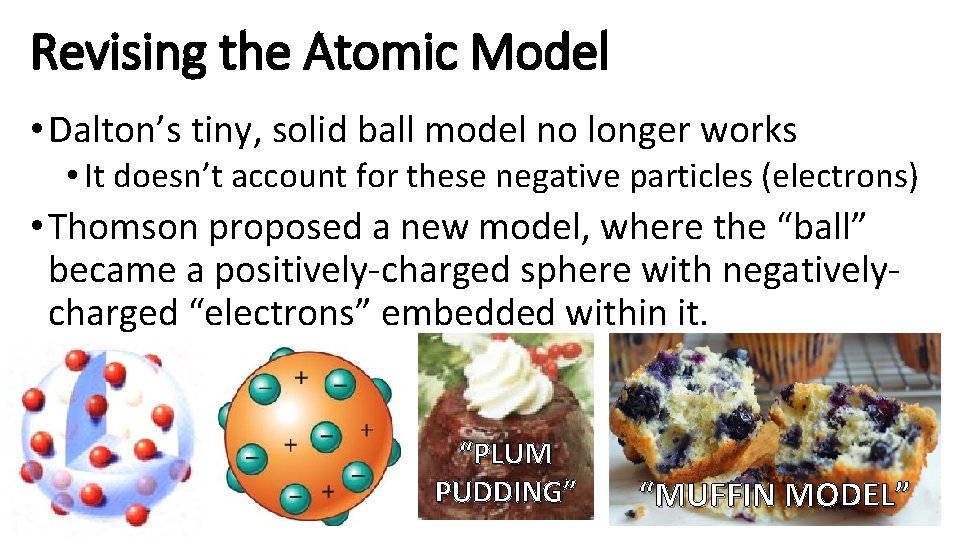
Revising the Atomic Model • Dalton’s tiny, solid ball model no longer works • It doesn’t account for these negative particles (electrons) • Thomson proposed a new model, where the “ball” became a positively-charged sphere with negativelycharged “electrons” embedded within it. “PLUM PUDDING” “MUFFIN MODEL”
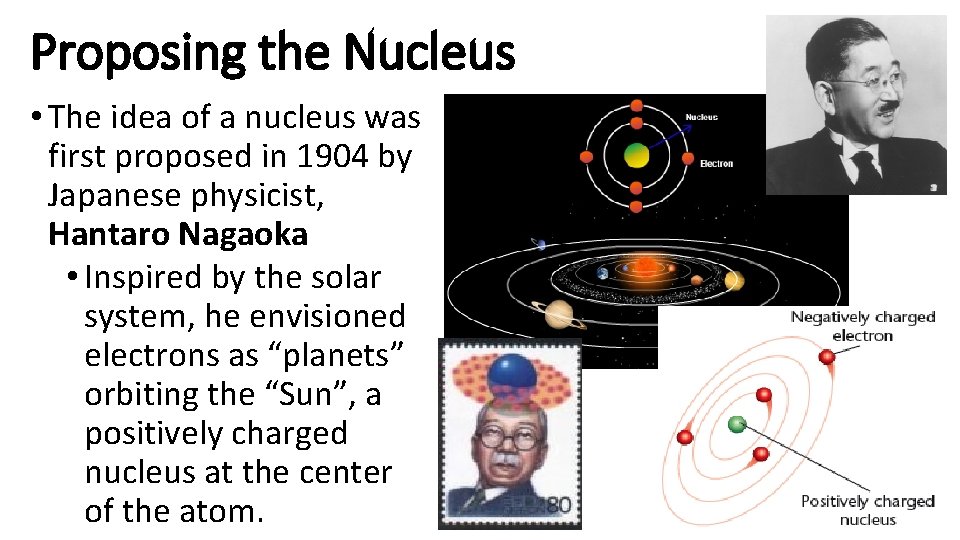
Proposing the Nucleus • The idea of a nucleus was first proposed in 1904 by Japanese physicist, Hantaro Nagaoka • Inspired by the solar system, he envisioned electrons as “planets” orbiting the “Sun”, a positively charged nucleus at the center of the atom.
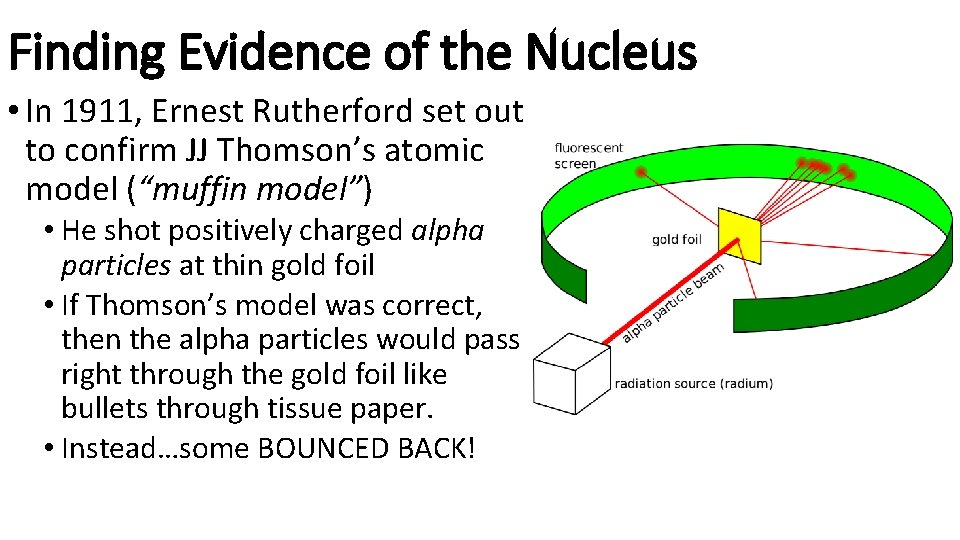
Finding Evidence of the Nucleus • In 1911, Ernest Rutherford set out to confirm JJ Thomson’s atomic model (“muffin model”) • He shot positively charged alpha particles at thin gold foil • If Thomson’s model was correct, then the alpha particles would pass right through the gold foil like bullets through tissue paper. • Instead…some BOUNCED BACK!
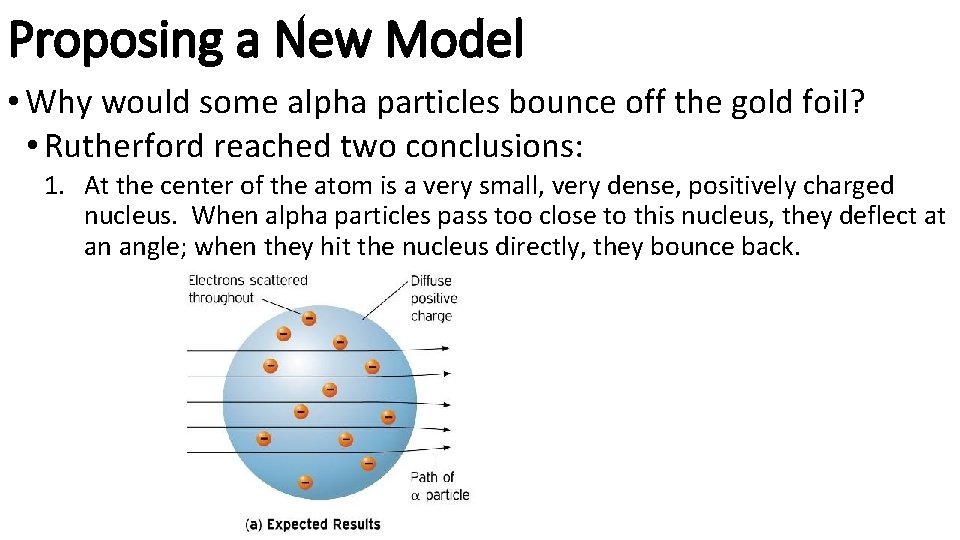
Proposing a New Model • Why would some alpha particles bounce off the gold foil? • Rutherford reached two conclusions: 1. At the center of the atom is a very small, very dense, positively charged nucleus. When alpha particles pass too close to this nucleus, they deflect at an angle; when they hit the nucleus directly, they bounce back.

Proposing a New Model • Why would the alpha particles bounce off the gold foil? • Rutherford reached two conclusions: 2. Most of the atom is empty space, allowing most of the alpha particles to pass right through the gold foil • If you remove the empty space in all the atoms in your body, you would shrink to a size smaller than a grain of salt…with the same mass you currently have. • If the nucleus is the size of a soccer ball, the nearest electron would be in orbit around the soccer ball a half-mile away, with only empty space in between.
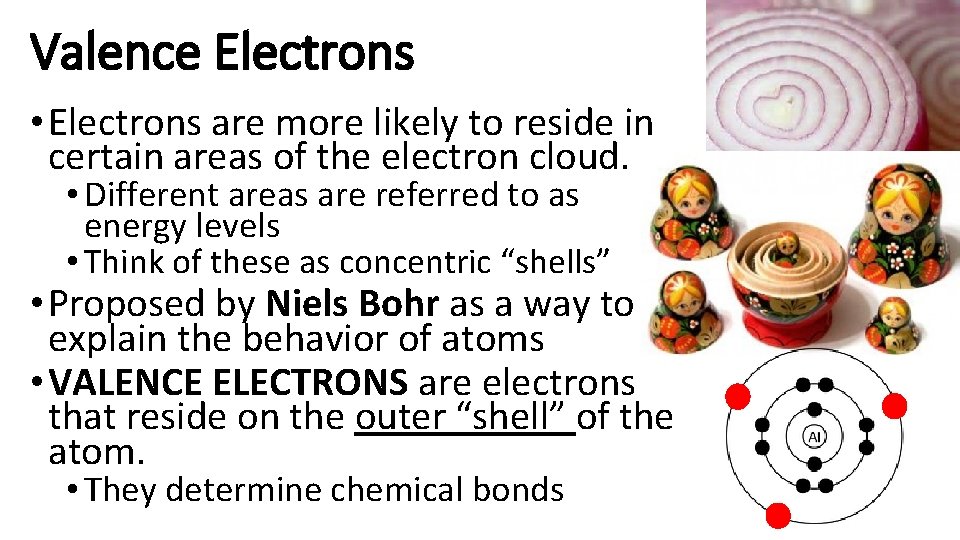
Valence Electrons • Electrons are more likely to reside in certain areas of the electron cloud. • Different areas are referred to as energy levels • Think of these as concentric “shells” • Proposed by Niels Bohr as a way to explain the behavior of atoms • VALENCE ELECTRONS are electrons that reside on the outer “shell” of the atom. • They determine chemical bonds

Discovering Protons • “Discovered” by Ernest Rutherford @1920 • Nucleus of a hydrogen atom is present in other nuclei • When alpha particles were shot at nitrogen, the nitrogen atoms absorbed them and emited hydrogen nuclei • Positively charged particles which reside in the nucleus • Charge = +1 (equal but opposite to electrons) • Mass = 2000 times greater than the mass of electrons • The number of protons in the nucleus is what makes each element unique

A Problem with Atomic Mass… • The mass of an atom could be predicted by the number of PROTONS and ELECTRONS it had… • It could be measured by comparing the weights of equal volumes of gasses (Atomic Mass Units) • THE PROBLEM… • The predictions did not match the measurements • The observed mass of atoms was always at least double what the prediction said it should be • Atomic physicists around the world struggled with how to explain the “extra mass” in atoms…

Neutrons • Discovered by James Chadwick in 1932 • Neutrons explained the “extra mass” • Since they had no charge, their mass is the only indication they are there. • Neutral particles which reside in the nucleus • Mass equal to the mass of a proton • No electrical charge
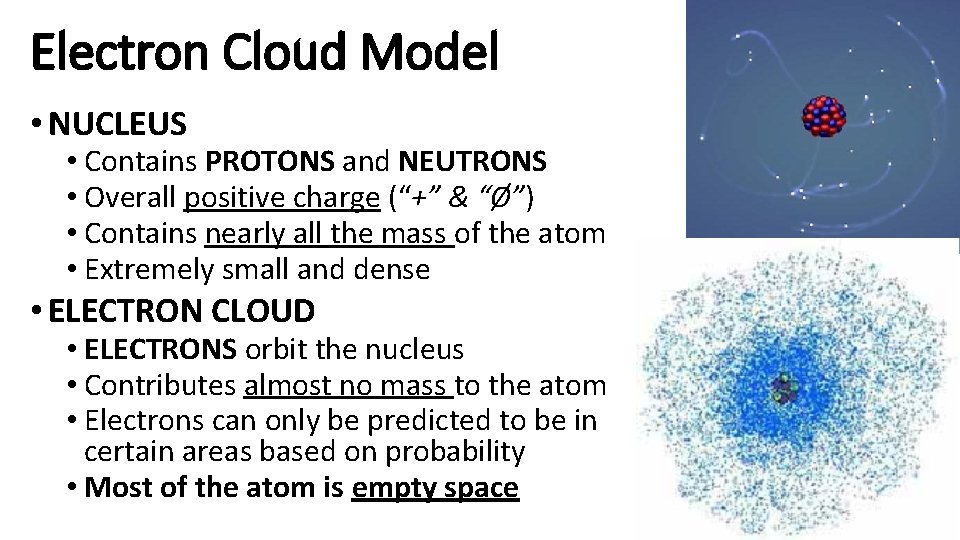
Electron Cloud Model • NUCLEUS • Contains PROTONS and NEUTRONS • Overall positive charge (“+” & “Ø”) • Contains nearly all the mass of the atom • Extremely small and dense • ELECTRON CLOUD • ELECTRONS orbit the nucleus • Contributes almost no mass to the atom • Electrons can only be predicted to be in certain areas based on probability • Most of the atom is empty space
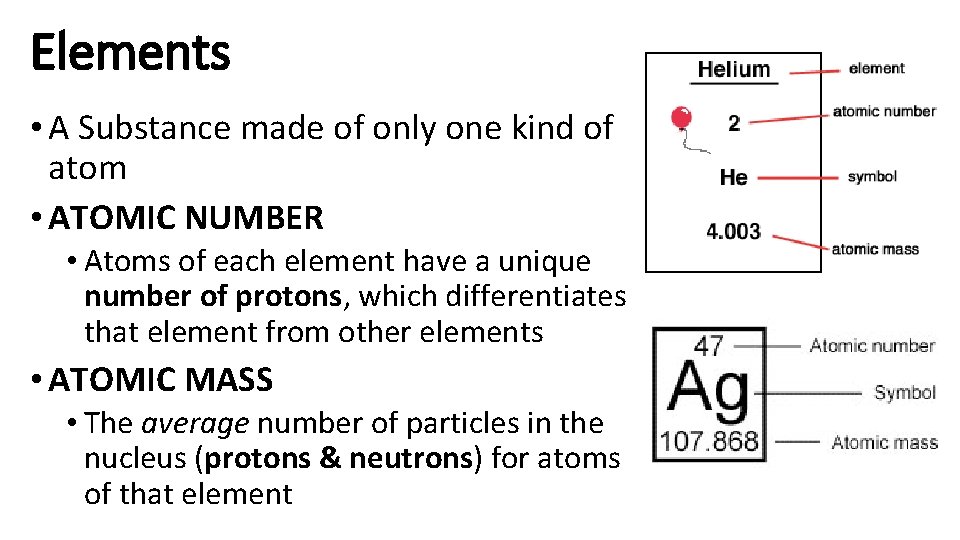
Elements • A Substance made of only one kind of atom • ATOMIC NUMBER • Atoms of each element have a unique number of protons, which differentiates that element from other elements • ATOMIC MASS • The average number of particles in the nucleus (protons & neutrons) for atoms of that element
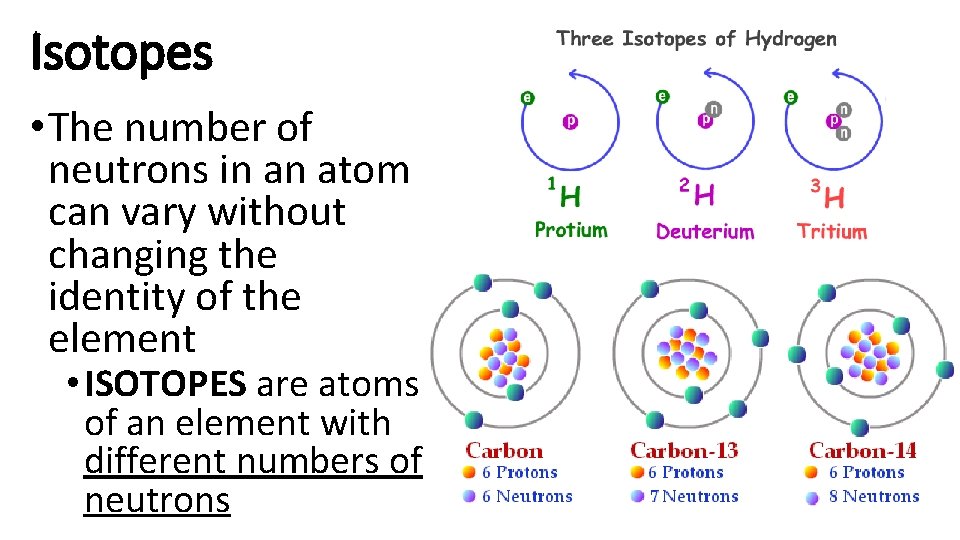
Isotopes • The number of neutrons in an atom can vary without changing the identity of the element • ISOTOPES are atoms of an element with different numbers of neutrons
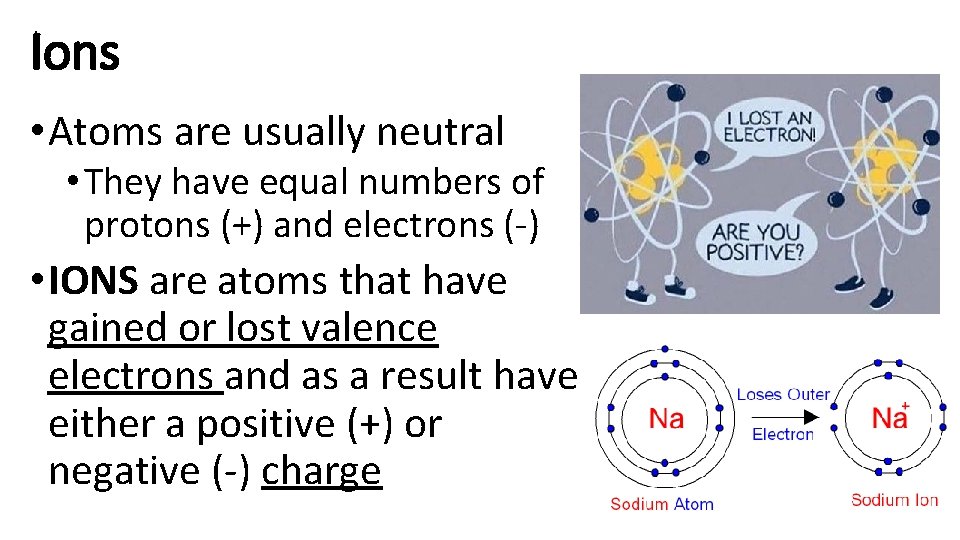
Ions • Atoms are usually neutral • They have equal numbers of protons (+) and electrons (-) • IONS are atoms that have gained or lost valence electrons and as a result have either a positive (+) or negative (-) charge
What if… …we start with a neutral atom? 12 CARBON 6 protons + 6 neutrons Ø 6 electrons - …we change the number of…? It will result in… PROTONS add one proton New Element NEUTRONS add one neutron Isotope ELECTRONS add one electron NITROGEN 7 p+ 6 n 0 6 e- Carbon 13 6 p+ 7 n 0 6 e- Ion Carbon- 6 p+ 6 n 0 7 e-
- Slides: 18