Chemistry SOL ReviewNomenclature Formulas and Reactions Chemistry SOL

- Slides: 36

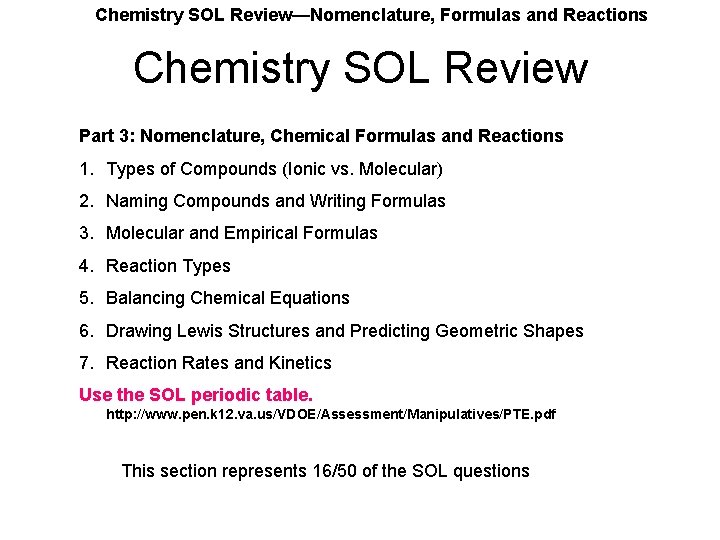
Chemistry SOL Review—Nomenclature, Formulas and Reactions Chemistry SOL Review Part 3: Nomenclature, Chemical Formulas and Reactions 1. Types of Compounds (Ionic vs. Molecular) 2. Naming Compounds and Writing Formulas 3. Molecular and Empirical Formulas 4. Reaction Types 5. Balancing Chemical Equations 6. Drawing Lewis Structures and Predicting Geometric Shapes 7. Reaction Rates and Kinetics Use the SOL periodic table. http: //www. pen. k 12. va. us/VDOE/Assessment/Manipulatives/PTE. pdf This section represents 16/50 of the SOL questions

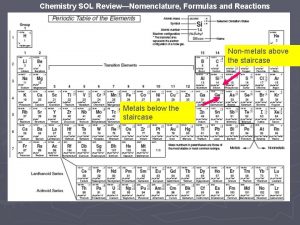
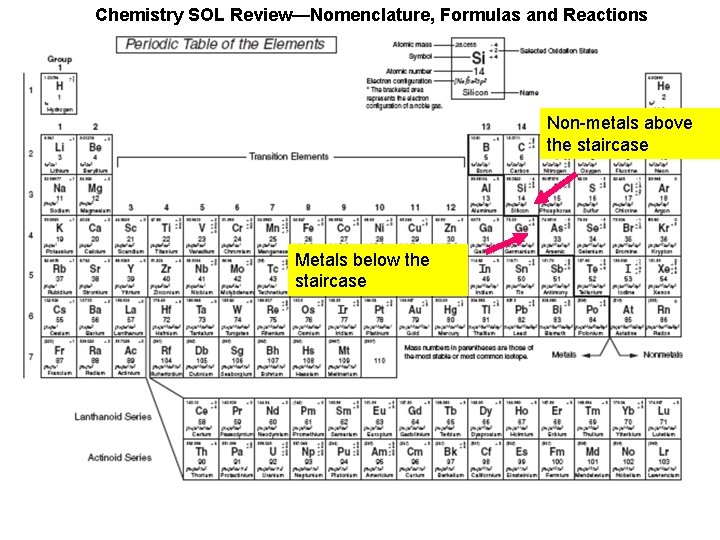
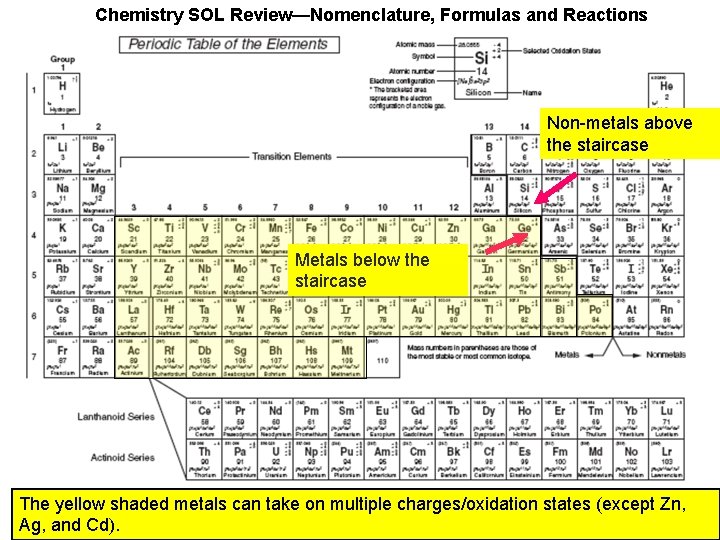
Chemistry SOL Review—Nomenclature, Formulas and Reactions Non-metals above the staircase Metals below the staircase

Chemistry SOL Review—Nomenclature, Formulas and Reactions Non-metals above the staircase Metals below the staircase The yellow shaded metals can take on multiple charges/oxidation states (except Zn, Ag, and Cd).

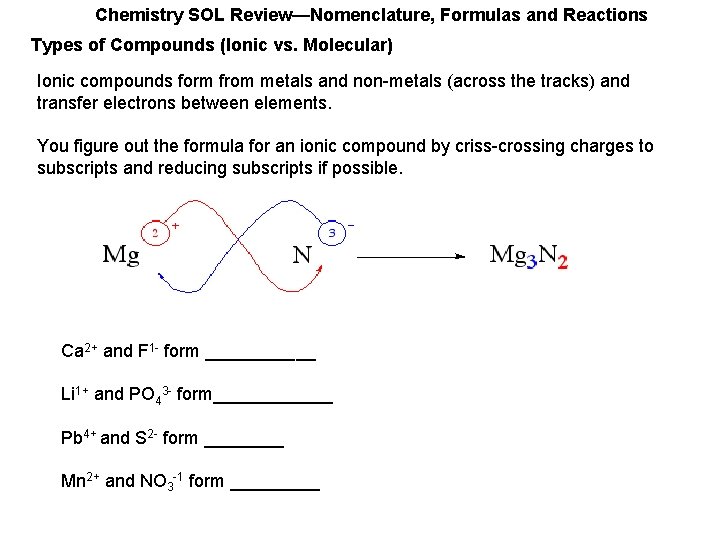
Chemistry SOL Review—Nomenclature, Formulas and Reactions Types of Compounds (Ionic vs. Molecular) Ionic compounds form from metals and non-metals (across the tracks) and transfer electrons between elements. You figure out the formula for an ionic compound by criss-crossing charges to subscripts and reducing subscripts if possible. Ca 2+ and F 1 - form ______ Li 1+ and PO 43 - form______ Pb 4+ and S 2 - form ____ Mn 2+ and NO 3 -1 form _____

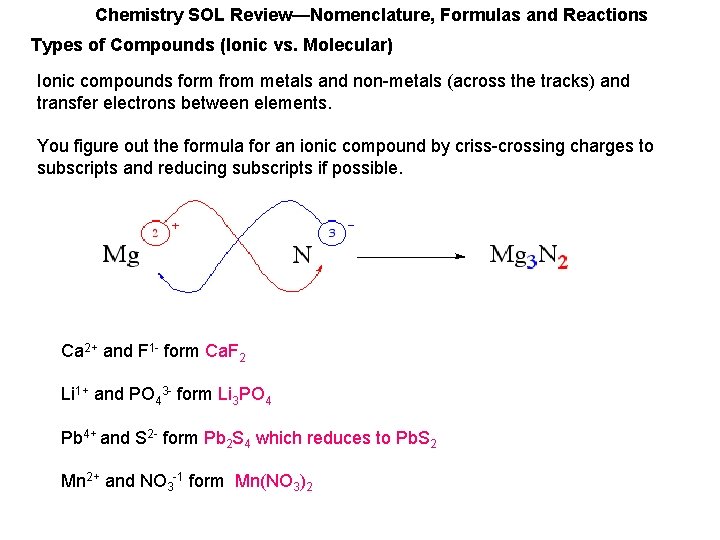
Chemistry SOL Review—Nomenclature, Formulas and Reactions Types of Compounds (Ionic vs. Molecular) Ionic compounds form from metals and non-metals (across the tracks) and transfer electrons between elements. You figure out the formula for an ionic compound by criss-crossing charges to subscripts and reducing subscripts if possible. Ca 2+ and F 1 - form Ca. F 2 Li 1+ and PO 43 - form Li 3 PO 4 Pb 4+ and S 2 - form Pb 2 S 4 which reduces to Pb. S 2 Mn 2+ and NO 3 -1 form Mn(NO 3)2

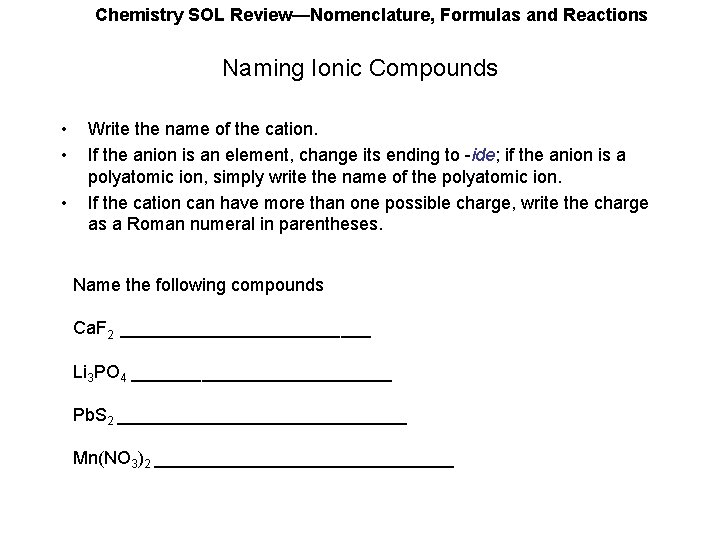
Chemistry SOL Review—Nomenclature, Formulas and Reactions Naming Ionic Compounds • • • Write the name of the cation. If the anion is an element, change its ending to -ide; if the anion is a polyatomic ion, simply write the name of the polyatomic ion. If the cation can have more than one possible charge, write the charge as a Roman numeral in parentheses. Name the following compounds Ca. F 2 _____________ Li 3 PO 4 _____________ Pb. S 2 _______________ Mn(NO 3)2 _______________

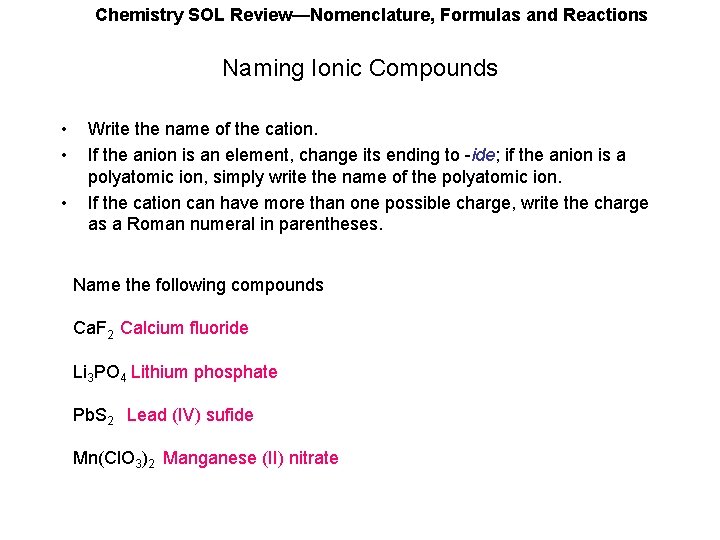
Chemistry SOL Review—Nomenclature, Formulas and Reactions Naming Ionic Compounds • • • Write the name of the cation. If the anion is an element, change its ending to -ide; if the anion is a polyatomic ion, simply write the name of the polyatomic ion. If the cation can have more than one possible charge, write the charge as a Roman numeral in parentheses. Name the following compounds Ca. F 2 Calcium fluoride Li 3 PO 4 Lithium phosphate Pb. S 2 Lead (IV) sufide Mn(Cl. O 3)2 Manganese (II) nitrate

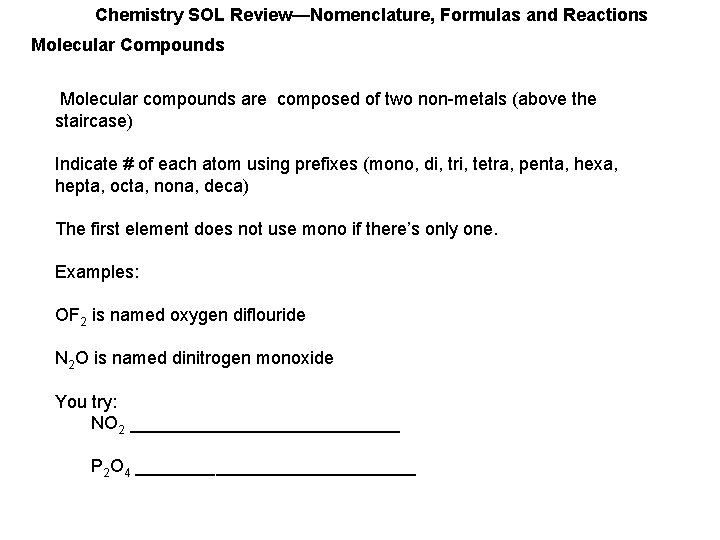
Chemistry SOL Review—Nomenclature, Formulas and Reactions Molecular Compounds Molecular compounds are composed of two non-metals (above the staircase) Indicate # of each atom using prefixes (mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca) The first element does not use mono if there’s only one. Examples: OF 2 is named oxygen diflouride N 2 O is named dinitrogen monoxide You try: NO 2 ______________ P 2 O 4 ______________

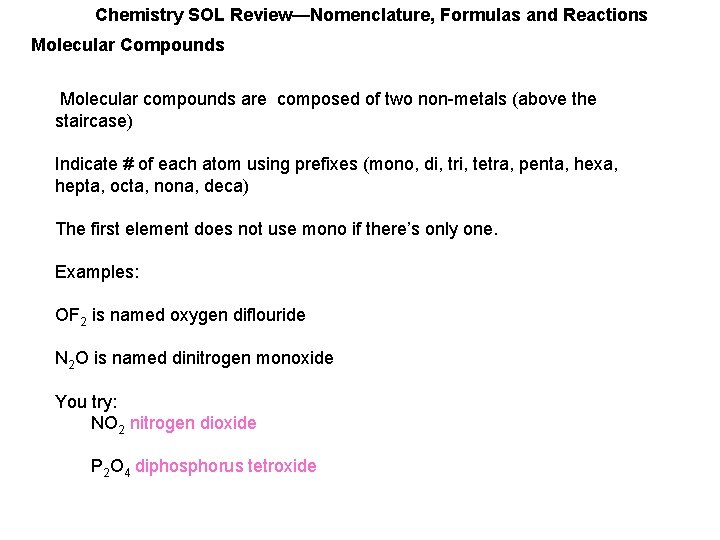
Chemistry SOL Review—Nomenclature, Formulas and Reactions Molecular Compounds Molecular compounds are composed of two non-metals (above the staircase) Indicate # of each atom using prefixes (mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca) The first element does not use mono if there’s only one. Examples: OF 2 is named oxygen diflouride N 2 O is named dinitrogen monoxide You try: NO 2 nitrogen dioxide P 2 O 4 diphosphorus tetroxide

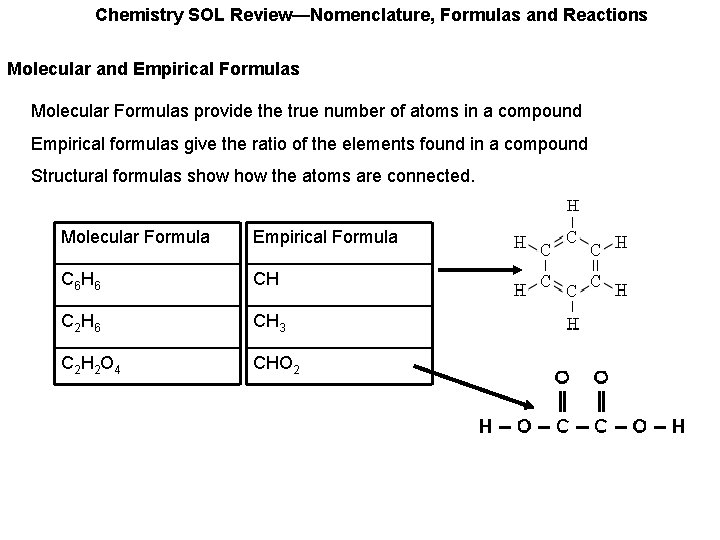
Chemistry SOL Review—Nomenclature, Formulas and Reactions Molecular and Empirical Formulas Molecular Formulas provide the true number of atoms in a compound Empirical formulas give the ratio of the elements found in a compound Structural formulas show the atoms are connected. Molecular Formula Empirical Formula C 6 H 6 CH C 2 H 6 CH 3 C 2 H 2 O 4 CHO 2

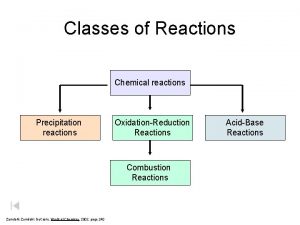
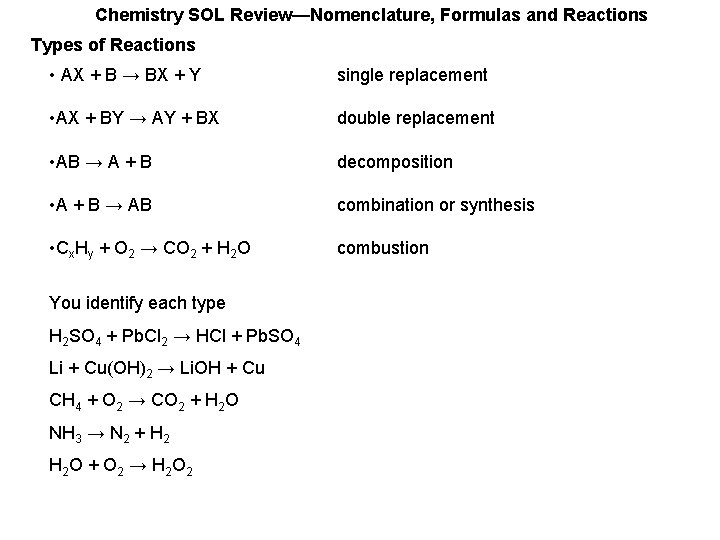
Chemistry SOL Review—Nomenclature, Formulas and Reactions Types of Reactions • AX + B → BX + Y single replacement • AX + BY → AY + BX double replacement • AB → A + B decomposition • A + B → AB combination or synthesis • Cx. Hy + O 2 → CO 2 + H 2 O combustion You identify each type H 2 SO 4 + Pb. Cl 2 → HCl + Pb. SO 4 Li + Cu(OH)2 → Li. OH + Cu CH 4 + O 2 → CO 2 + H 2 O NH 3 → N 2 + H 2 O + O 2 → H 2 O 2

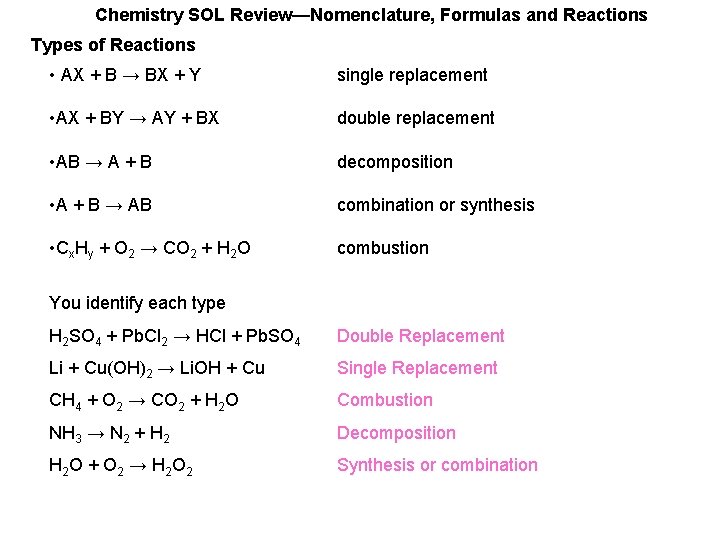
Chemistry SOL Review—Nomenclature, Formulas and Reactions Types of Reactions • AX + B → BX + Y single replacement • AX + BY → AY + BX double replacement • AB → A + B decomposition • A + B → AB combination or synthesis • Cx. Hy + O 2 → CO 2 + H 2 O combustion You identify each type H 2 SO 4 + Pb. Cl 2 → HCl + Pb. SO 4 Double Replacement Li + Cu(OH)2 → Li. OH + Cu Single Replacement CH 4 + O 2 → CO 2 + H 2 O Combustion NH 3 → N 2 + H 2 Decomposition H 2 O + O 2 → H 2 O 2 Synthesis or combination

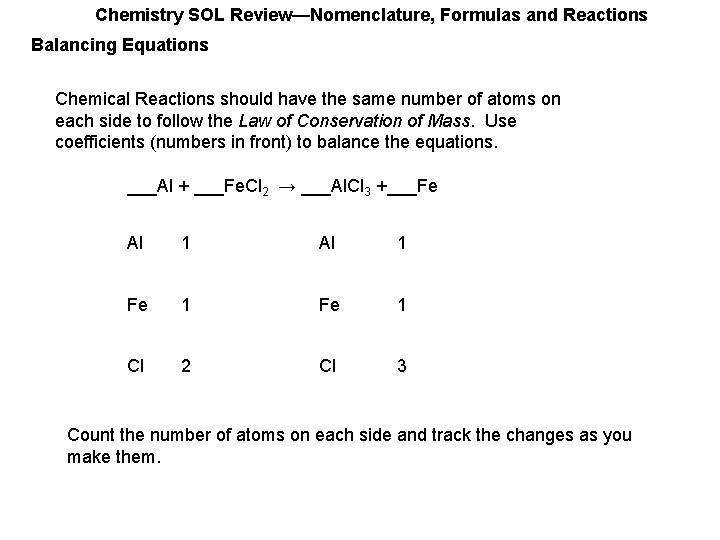
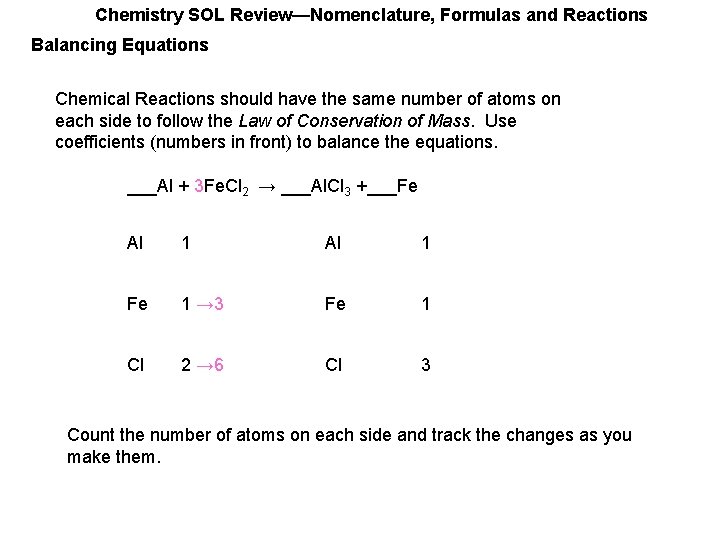
Chemistry SOL Review—Nomenclature, Formulas and Reactions Balancing Equations Chemical Reactions should have the same number of atoms on each side to follow the Law of Conservation of Mass. Use coefficients (numbers in front) to balance the equations. ___Al + ___Fe. Cl 2 → ___Al. Cl 3 +___Fe Al 1 Fe 1 Cl 2 Cl 3 Count the number of atoms on each side and track the changes as you make them.

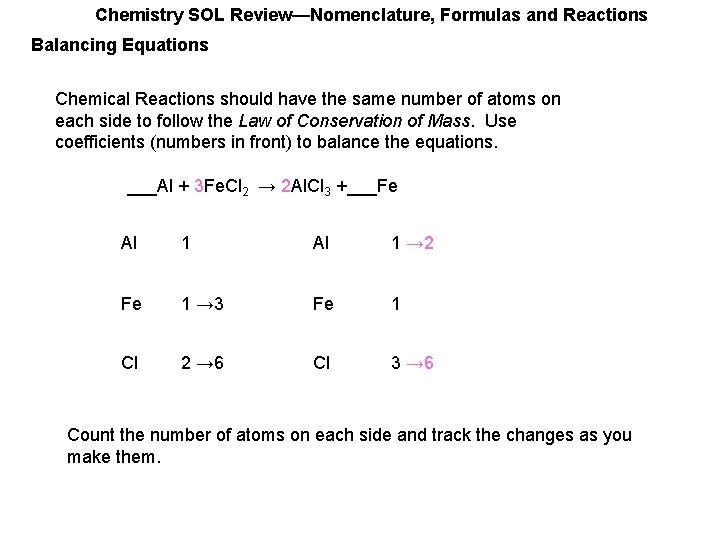
Chemistry SOL Review—Nomenclature, Formulas and Reactions Balancing Equations Chemical Reactions should have the same number of atoms on each side to follow the Law of Conservation of Mass. Use coefficients (numbers in front) to balance the equations. ___Al + 3 Fe. Cl 2 → ___Al. Cl 3 +___Fe Al 1 Fe 1 → 3 Fe 1 Cl 2 → 6 Cl 3 Count the number of atoms on each side and track the changes as you make them.

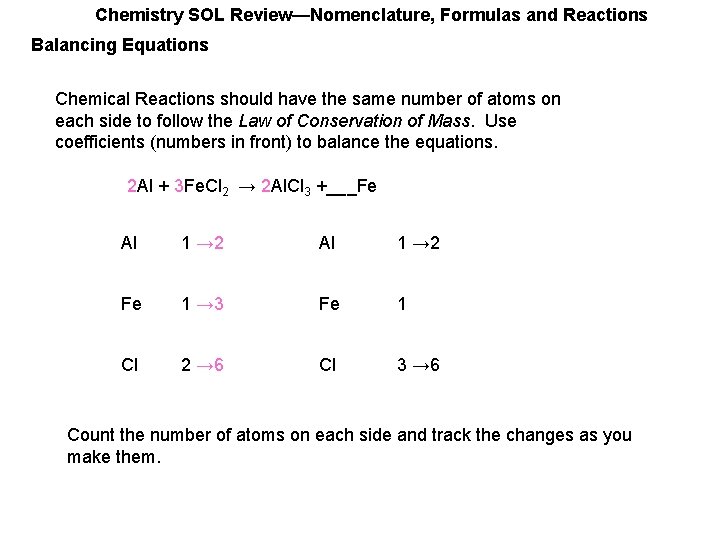
Chemistry SOL Review—Nomenclature, Formulas and Reactions Balancing Equations Chemical Reactions should have the same number of atoms on each side to follow the Law of Conservation of Mass. Use coefficients (numbers in front) to balance the equations. ___Al + 3 Fe. Cl 2 → 2 Al. Cl 3 +___Fe Al 1 → 2 Fe 1 → 3 Fe 1 Cl 2 → 6 Cl 3 → 6 Count the number of atoms on each side and track the changes as you make them.

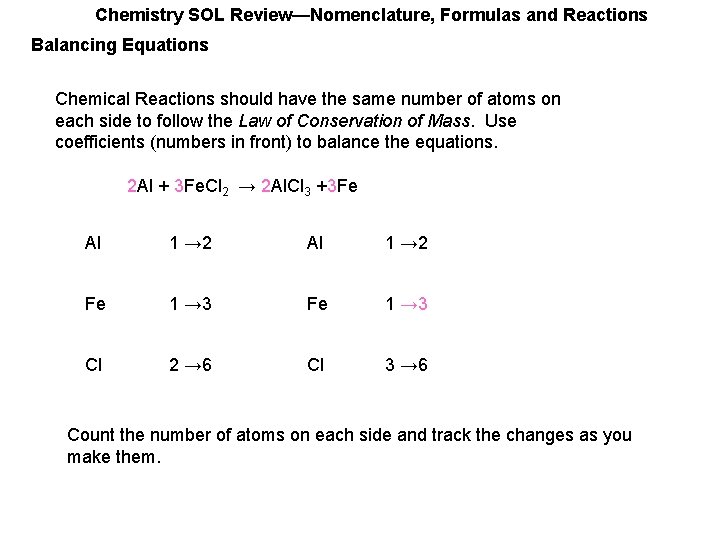
Chemistry SOL Review—Nomenclature, Formulas and Reactions Balancing Equations Chemical Reactions should have the same number of atoms on each side to follow the Law of Conservation of Mass. Use coefficients (numbers in front) to balance the equations. 2 Al + 3 Fe. Cl 2 → 2 Al. Cl 3 +___Fe Al 1 → 2 Fe 1 → 3 Fe 1 Cl 2 → 6 Cl 3 → 6 Count the number of atoms on each side and track the changes as you make them.

Chemistry SOL Review—Nomenclature, Formulas and Reactions Balancing Equations Chemical Reactions should have the same number of atoms on each side to follow the Law of Conservation of Mass. Use coefficients (numbers in front) to balance the equations. 2 Al + 3 Fe. Cl 2 → 2 Al. Cl 3 +3 Fe Al 1 → 2 Fe 1 → 3 Cl 2 → 6 Cl 3 → 6 Count the number of atoms on each side and track the changes as you make them.

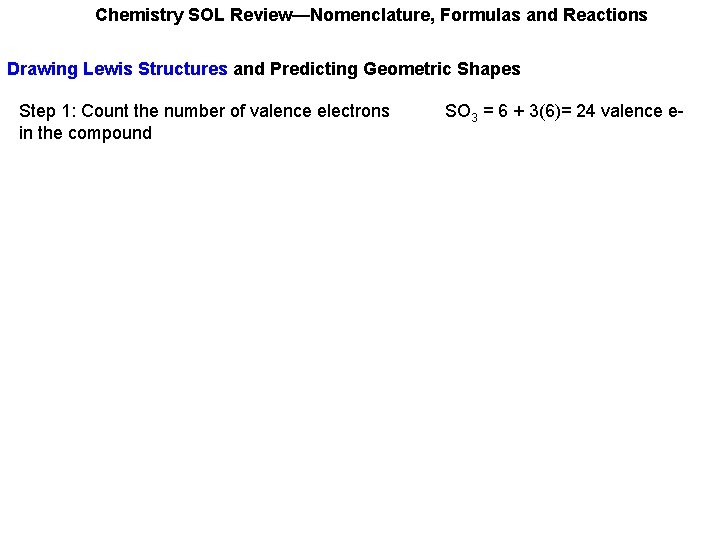
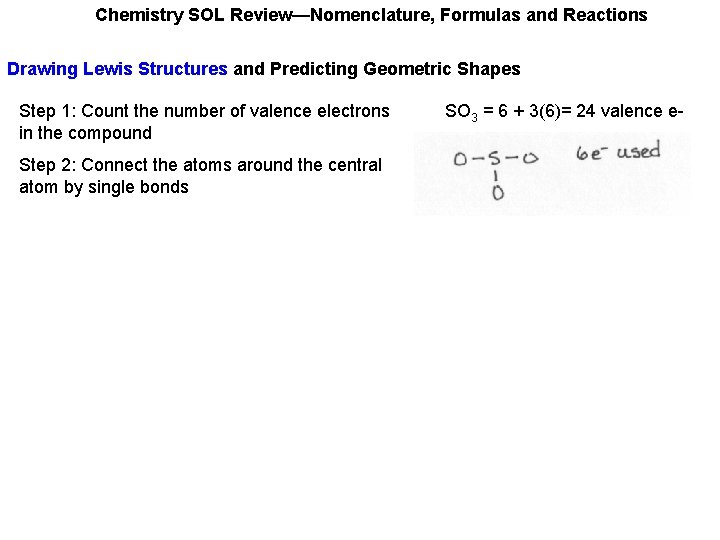
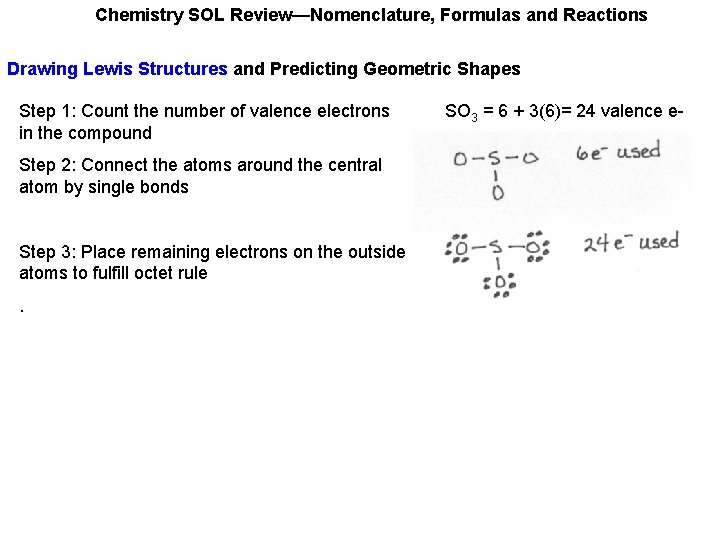
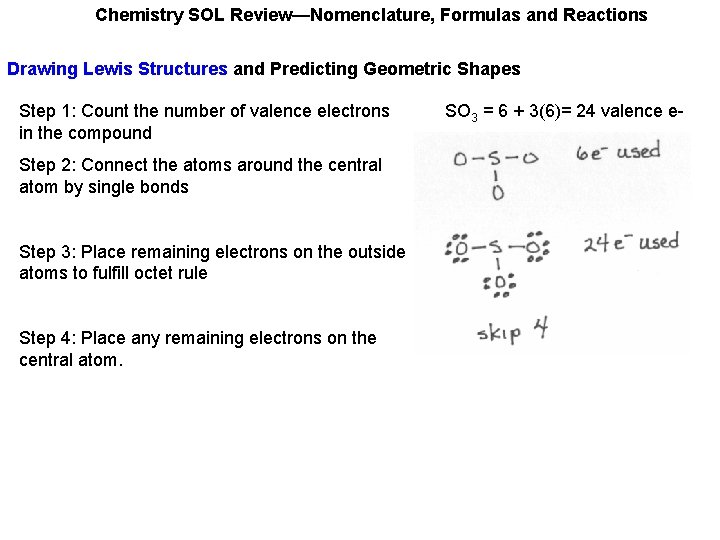
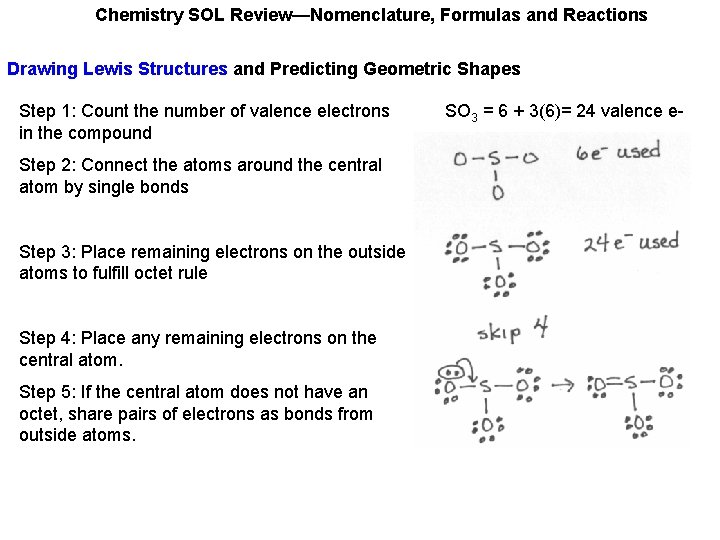
Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound SO 3 = 6 + 3(6)= 24 valence e-

Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound Step 2: Connect the atoms around the central atom by single bonds SO 3 = 6 + 3(6)= 24 valence e-

Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound Step 2: Connect the atoms around the central atom by single bonds Step 3: Place remaining electrons on the outside atoms to fulfill octet rule. SO 3 = 6 + 3(6)= 24 valence e-

Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound Step 2: Connect the atoms around the central atom by single bonds Step 3: Place remaining electrons on the outside atoms to fulfill octet rule Step 4: Place any remaining electrons on the central atom. SO 3 = 6 + 3(6)= 24 valence e-

Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound Step 2: Connect the atoms around the central atom by single bonds Step 3: Place remaining electrons on the outside atoms to fulfill octet rule Step 4: Place any remaining electrons on the central atom. Step 5: If the central atom does not have an octet, share pairs of electrons as bonds from outside atoms. SO 3 = 6 + 3(6)= 24 valence e-

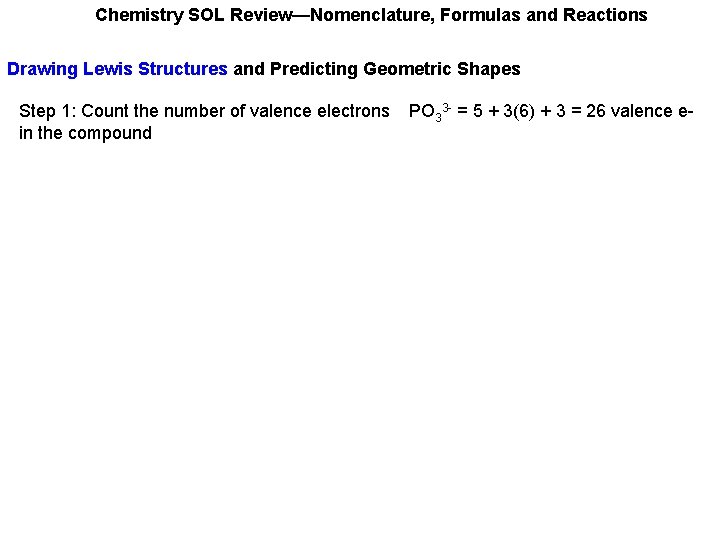
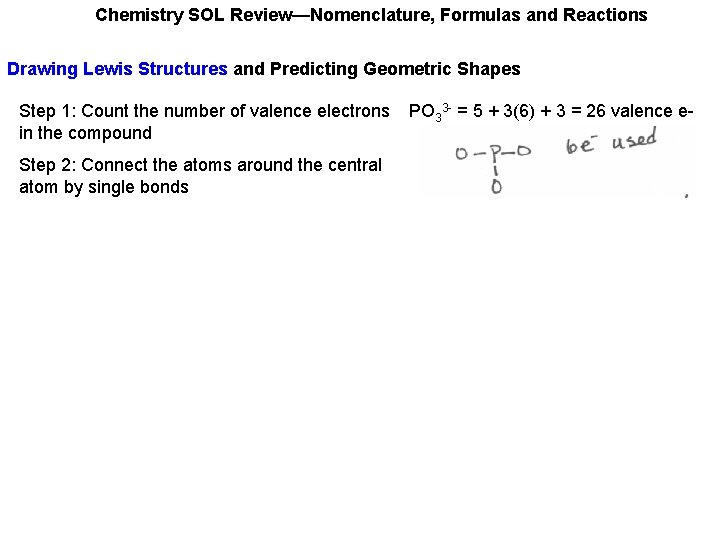
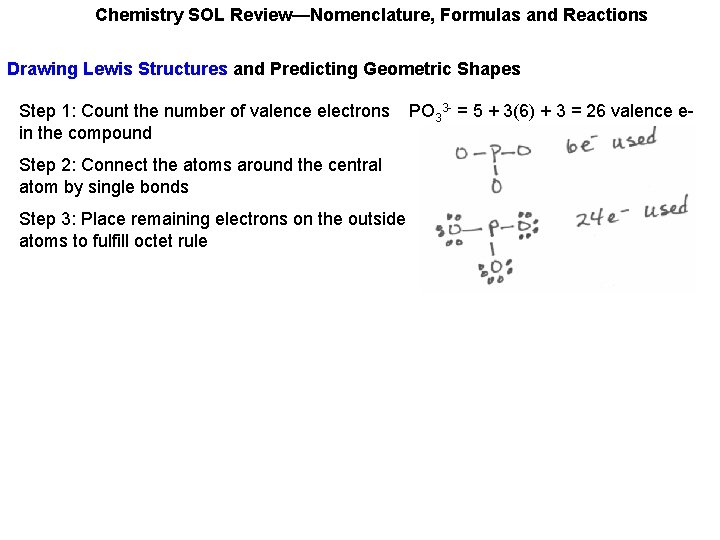
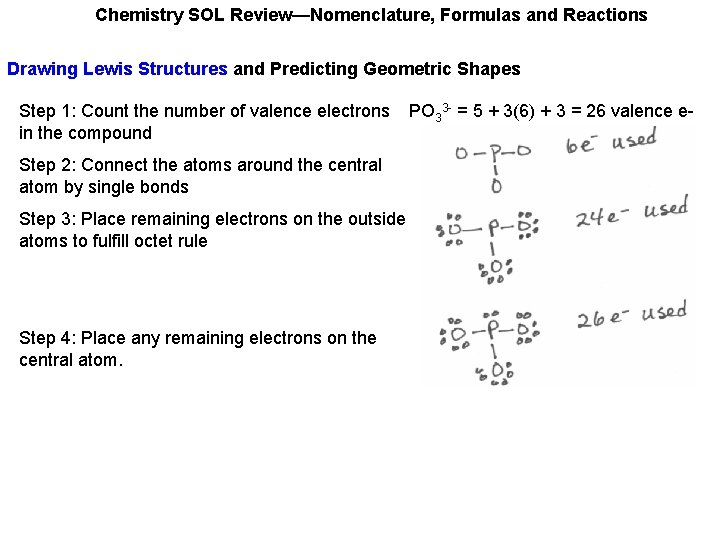
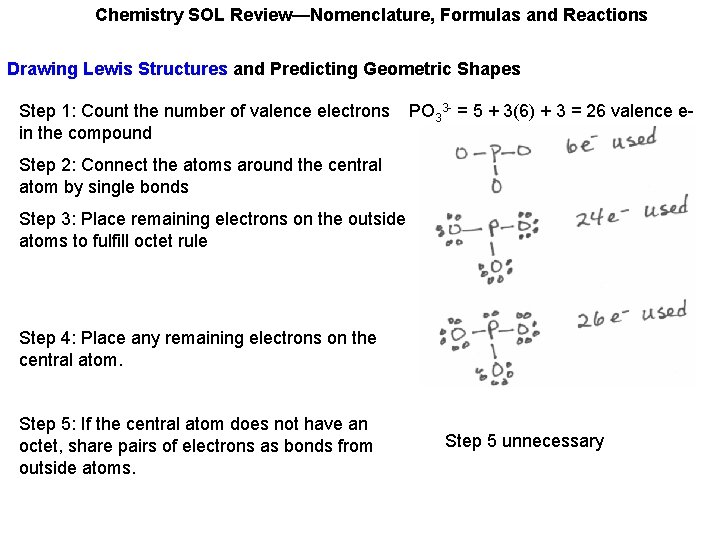
Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound PO 33 - = 5 + 3(6) + 3 = 26 valence e-

Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound Step 2: Connect the atoms around the central atom by single bonds PO 33 - = 5 + 3(6) + 3 = 26 valence e-

Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound Step 2: Connect the atoms around the central atom by single bonds Step 3: Place remaining electrons on the outside atoms to fulfill octet rule PO 33 - = 5 + 3(6) + 3 = 26 valence e-

Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound Step 2: Connect the atoms around the central atom by single bonds Step 3: Place remaining electrons on the outside atoms to fulfill octet rule Step 4: Place any remaining electrons on the central atom. PO 33 - = 5 + 3(6) + 3 = 26 valence e-

Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound PO 33 - = 5 + 3(6) + 3 = 26 valence e- Step 2: Connect the atoms around the central atom by single bonds Step 3: Place remaining electrons on the outside atoms to fulfill octet rule Step 4: Place any remaining electrons on the central atom. Step 5: If the central atom does not have an octet, share pairs of electrons as bonds from outside atoms. Step 5 unnecessary

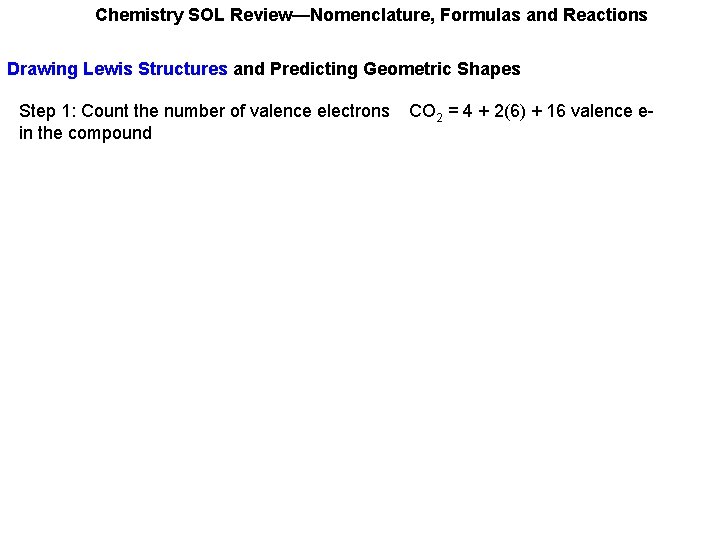
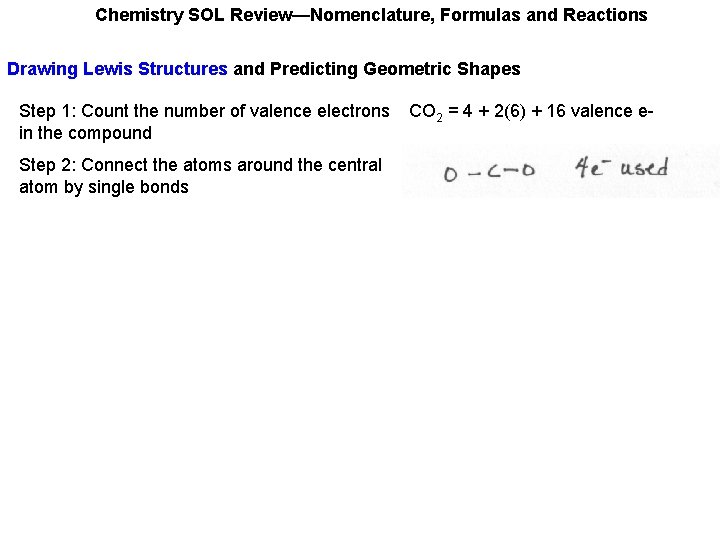
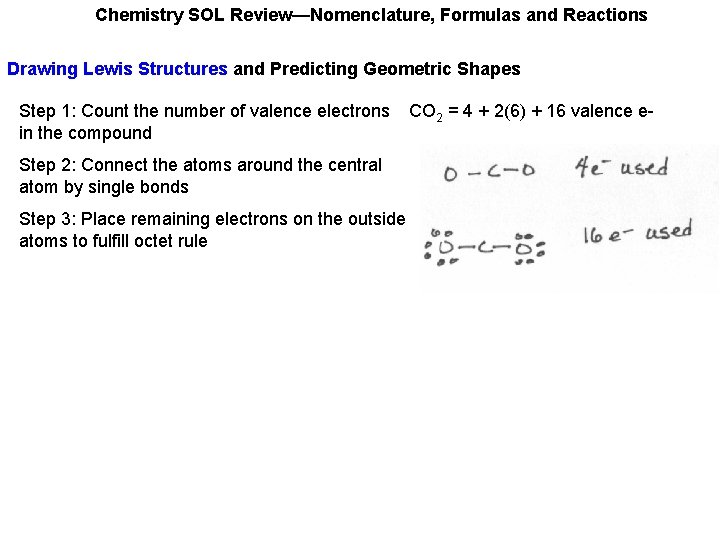
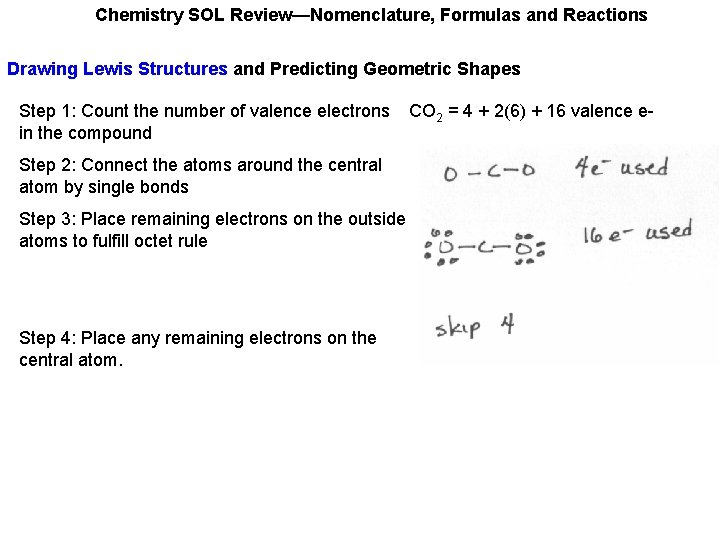
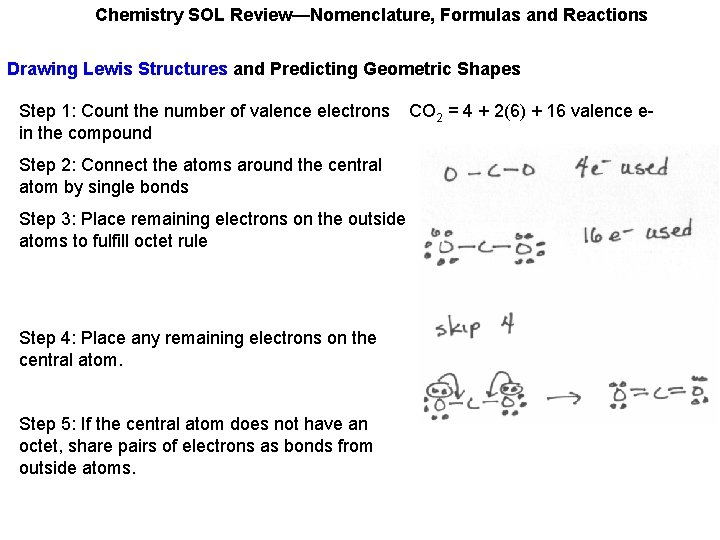
Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound CO 2 = 4 + 2(6) + 16 valence e-

Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound Step 2: Connect the atoms around the central atom by single bonds CO 2 = 4 + 2(6) + 16 valence e-

Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound Step 2: Connect the atoms around the central atom by single bonds Step 3: Place remaining electrons on the outside atoms to fulfill octet rule CO 2 = 4 + 2(6) + 16 valence e-

Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound Step 2: Connect the atoms around the central atom by single bonds Step 3: Place remaining electrons on the outside atoms to fulfill octet rule Step 4: Place any remaining electrons on the central atom. CO 2 = 4 + 2(6) + 16 valence e-

Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Step 1: Count the number of valence electrons in the compound Step 2: Connect the atoms around the central atom by single bonds Step 3: Place remaining electrons on the outside atoms to fulfill octet rule Step 4: Place any remaining electrons on the central atom. Step 5: If the central atom does not have an octet, share pairs of electrons as bonds from outside atoms. CO 2 = 4 + 2(6) + 16 valence e-

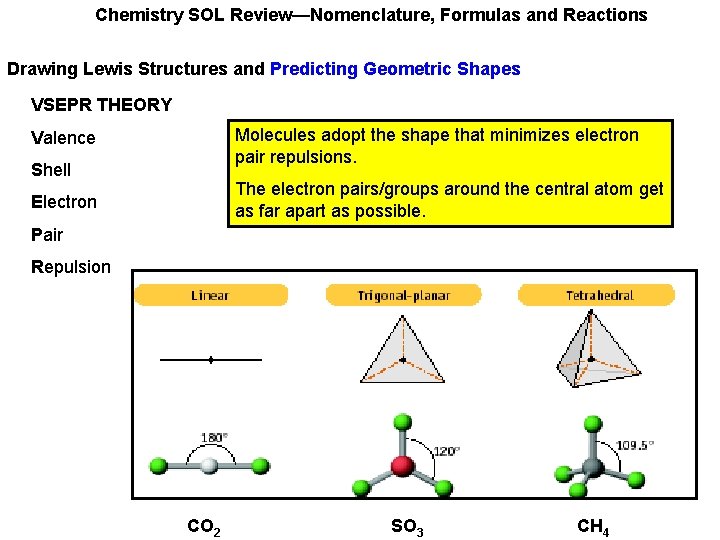
Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes VSEPR THEORY Molecules adopt the shape that minimizes electron pair repulsions. Valence Shell The electron pairs/groups around the central atom get as far apart as possible. Electron Pair Repulsion CO 2 SO 3 CH 4

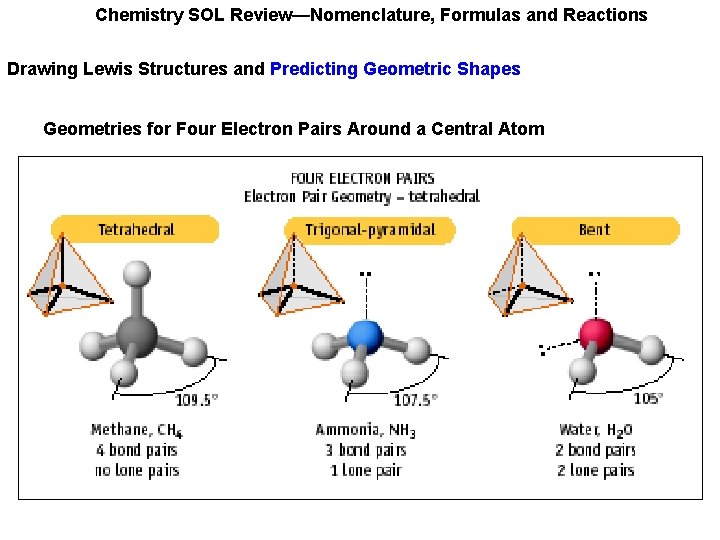
Chemistry SOL Review—Nomenclature, Formulas and Reactions Drawing Lewis Structures and Predicting Geometric Shapes Geometries for Four Electron Pairs Around a Central Atom

Chemistry SOL Review—Nomenclature, Formulas and Reactions Reaction Rates and Kinetics Ways to speed up reactions: 1. Decrease particle size (grind the sample) 2. Increase heat 3. Add catalyst 4. Increase concentration of reagents

Chemistry SOL Review—Nomenclature, Formulas and Reactions References http: //www. markrosengarten. com/ for New York Regent’s exam powerpoint.
 Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers How to write half reactions
How to write half reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Contoh koloid liofil
Contoh koloid liofil Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas 4 types of chemical reactions
4 types of chemical reactions 5 types of reactions in chemistry
5 types of reactions in chemistry Type of reactions chemistry
Type of reactions chemistry Reaction type synthesis
Reaction type synthesis Chemistry reactions
Chemistry reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 chemistry in biology
Chapter 6 chemistry in biology Organic chemistry formulas
Organic chemistry formulas Science formula list
Science formula list Chemistry sol review packet
Chemistry sol review packet Sol'n chemistry
Sol'n chemistry Kuhinjska sol formula
Kuhinjska sol formula Chemistry sol review
Chemistry sol review Chemistry sol review
Chemistry sol review Chemistry sol review
Chemistry sol review Chemistry sol review
Chemistry sol review Chemistry sol review
Chemistry sol review Ib organic chemistry functional groups
Ib organic chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Naming and writing formulas for acids and bases
Naming and writing formulas for acids and bases Naming and writing formulas for acids and bases
Naming and writing formulas for acids and bases Nuclear decays and reactions section 2
Nuclear decays and reactions section 2 Reversible and irreversible reactions
Reversible and irreversible reactions Oxidize vs reduce
Oxidize vs reduce What is a half reaction
What is a half reaction Reactants and products
Reactants and products Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes