Atomic Theory Review Basic Atomic Structure A Look

Atomic Theory Review Basic Atomic Structure: A Look Inside the Atom Watch the Video Linked above Before starting these notes.
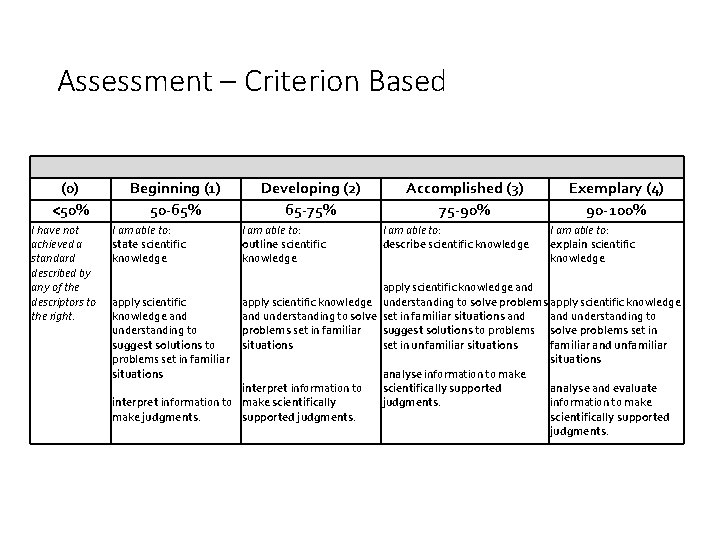
Assessment – Criterion Based (0) <50% I have not achieved a standard described by any of the descriptors to the right. Beginning (1) 50 -65% I am able to: state scientific knowledge Developing (2) 65 -75% I am able to: outline scientific knowledge Accomplished (3) 75 -90% I am able to: describe scientific knowledge Exemplary (4) 90 -100% I am able to: explain scientific knowledge apply scientific knowledge and apply scientific knowledge understanding to solve problems apply scientific knowledge and understanding to solve set in familiar situations and understanding to problems set in familiar suggest solutions to problems solve problems set in suggest solutions to situations set in unfamiliar situations familiar and unfamiliar problems set in familiar situations analyse information to make scientifically supported analyse and evaluate interpret information to make scientifically judgments. information to make judgments. scientifically supported judgments.
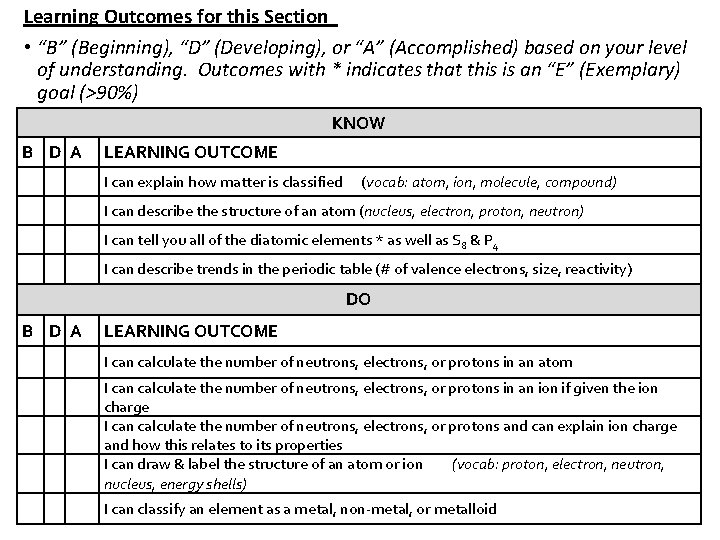
Learning Outcomes for this Section • “B” (Beginning), “D” (Developing), or “A” (Accomplished) based on your level of understanding. Outcomes with * indicates that this is an “E” (Exemplary) goal (>90%) KNOW B D A LEARNING OUTCOME I can explain how matter is classified (vocab: atom, ion, molecule, compound) I can describe the structure of an atom (nucleus, electron, proton, neutron) I can tell you all of the diatomic elements * as well as S 8 & P 4 I can describe trends in the periodic table (# of valence electrons, size, reactivity) DO B D A LEARNING OUTCOME I can calculate the number of neutrons, electrons, or protons in an atom I can calculate the number of neutrons, electrons, or protons in an ion if given the ion charge I can calculate the number of neutrons, electrons, or protons and can explain ion charge and how this relates to its properties I can draw & label the structure of an atom or ion (vocab: proton, electron, neutron, nucleus, energy shells) I can classify an element as a metal, non-metal, or metalloid
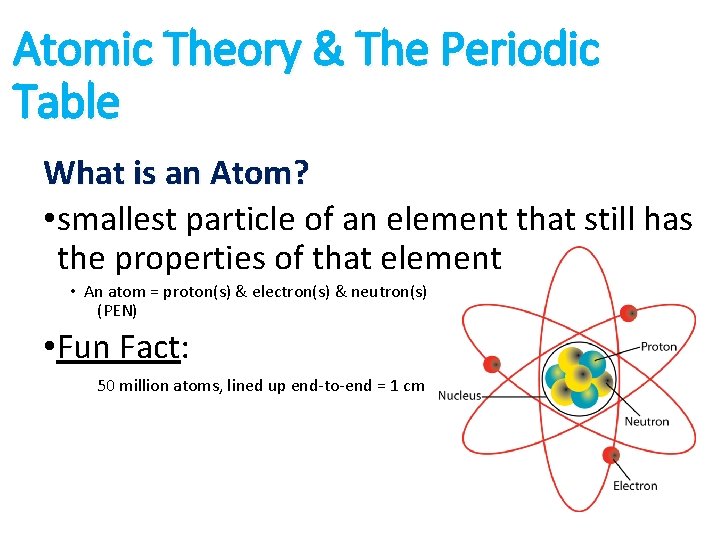
Atomic Theory & The Periodic Table What is an Atom? • smallest particle of an element that still has the properties of that element • An atom = proton(s) & electron(s) & neutron(s) (PEN) • Fun Fact: 50 million atoms, lined up end-to-end = 1 cm
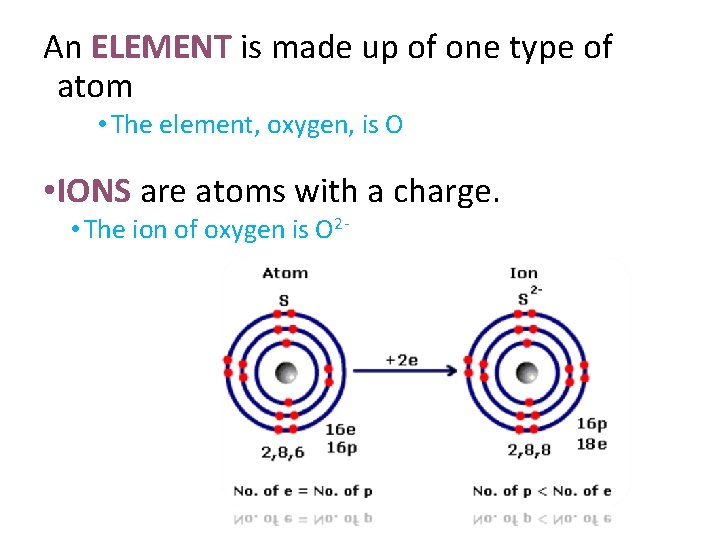
An ELEMENT is made up of one type of atom • The element, oxygen, is O • IONS are atoms with a charge. • The ion of oxygen is O 2 -
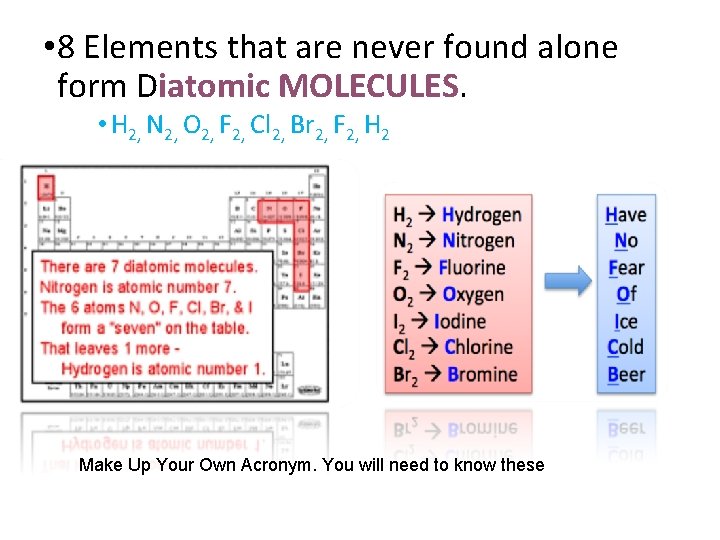
• 8 Elements that are never found alone form Diatomic MOLECULES. • H 2, N 2, O 2, F 2, Cl 2, Br 2, F 2, H 2 Make Up Your Own Acronym. You will need to know these
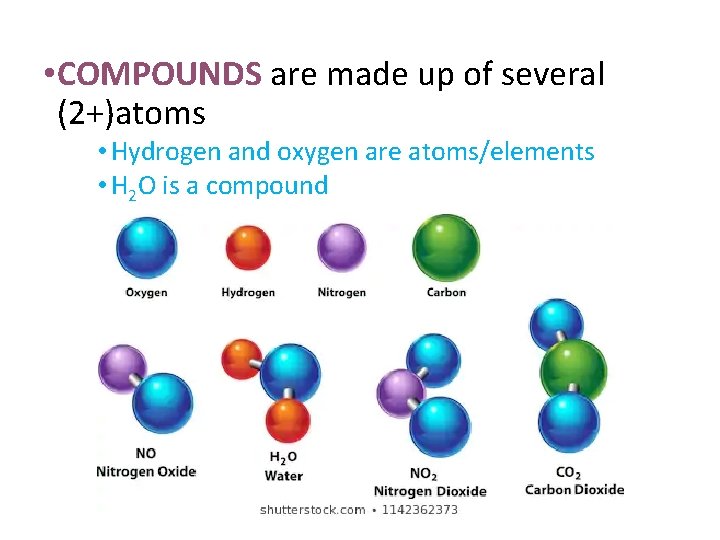
• COMPOUNDS are made up of several (2+)atoms • Hydrogen and oxygen are atoms/elements • H 2 O is a compound
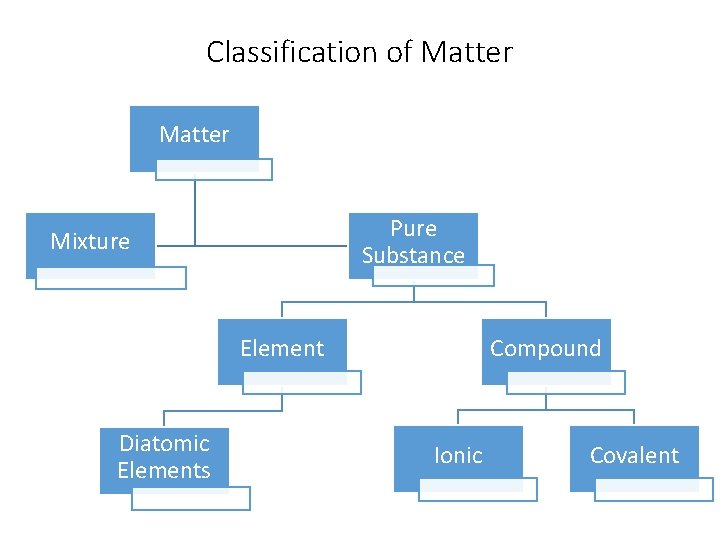
Classification of Matter Pure Substance Mixture Element Diatomic Elements Compound Ionic Covalent
Classification of Matter Mixture Diatomic Elements • Has mass and volume • Exists in 3 states; solid, liquid, gas Pure Substance e t a t o Elementn Compound w n A Dra les p m Ionic Covalent a x E
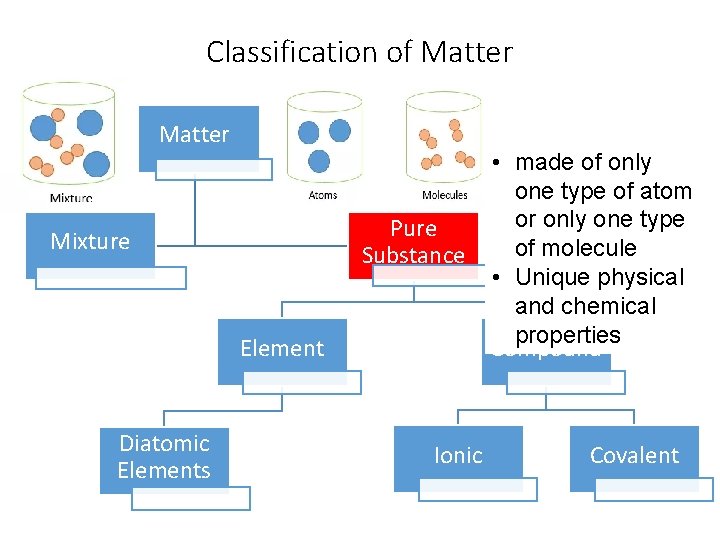
Classification of Matter Pure Substance Mixture Element Diatomic Elements Ionic • made of only one type of atom or only one type of molecule • Unique physical and chemical properties Compound Covalent
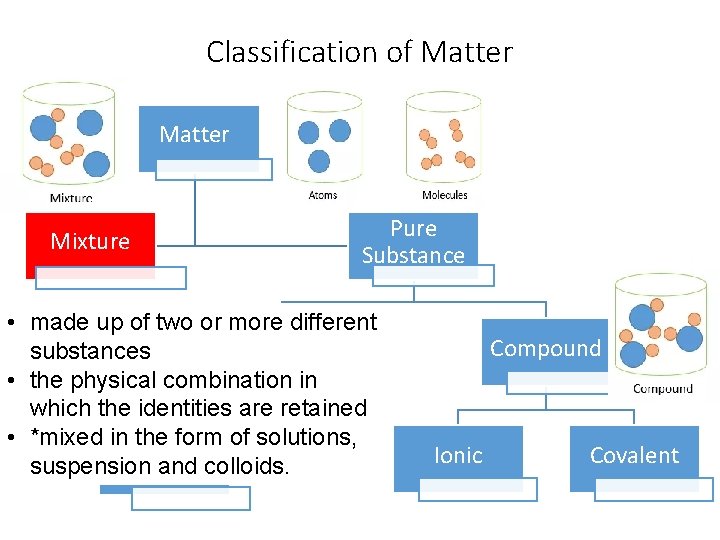
Classification of Matter Mixture Pure Substance • made up of two or more different Element substances • the physical combination in which the identities are retained • *mixed in. Diatomic the form of solutions, suspension and colloids. Elements Compound Ionic Covalent
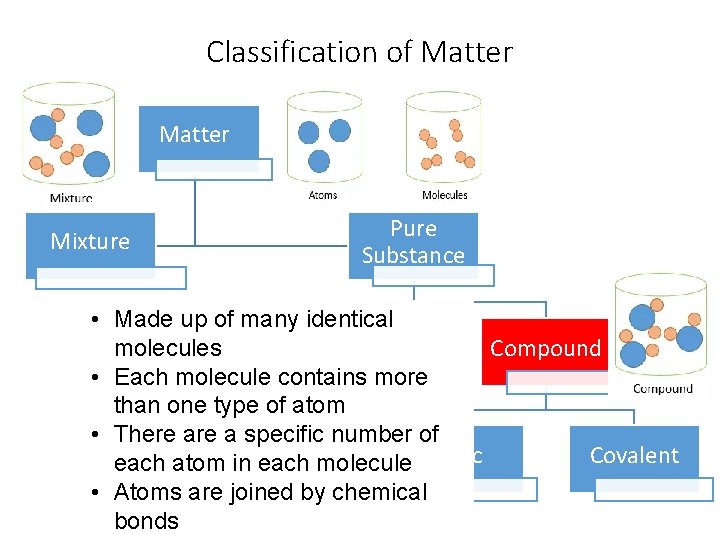
Classification of Matter Mixture Pure Substance • Made up of many identical Compound molecules Element • Each molecule contains more than one type of atom • There a specific number of Diatomic Ionic Covalent each atom in each molecule Elements • Atoms are joined by chemical bonds
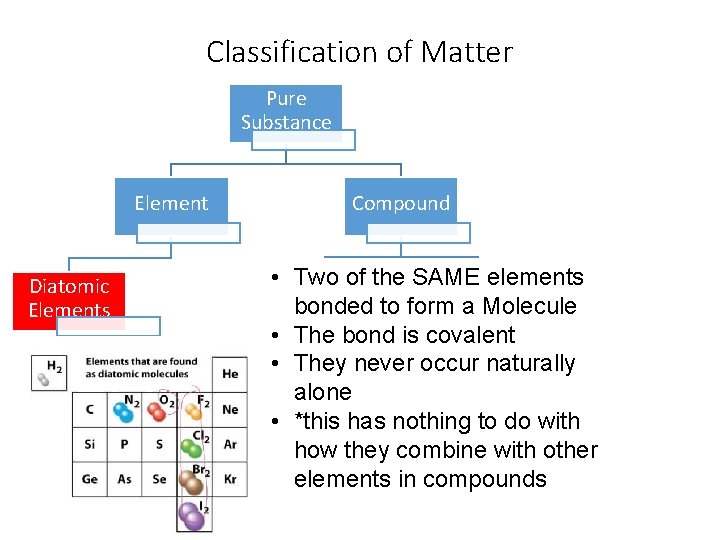
Classification of Matter Pure Substance Element Diatomic Elements Compound • Two of the SAME elements Ionic Covalent bonded to form a Molecule • The bond is covalent • They never occur naturally alone • *this has nothing to do with how they combine with other elements in compounds
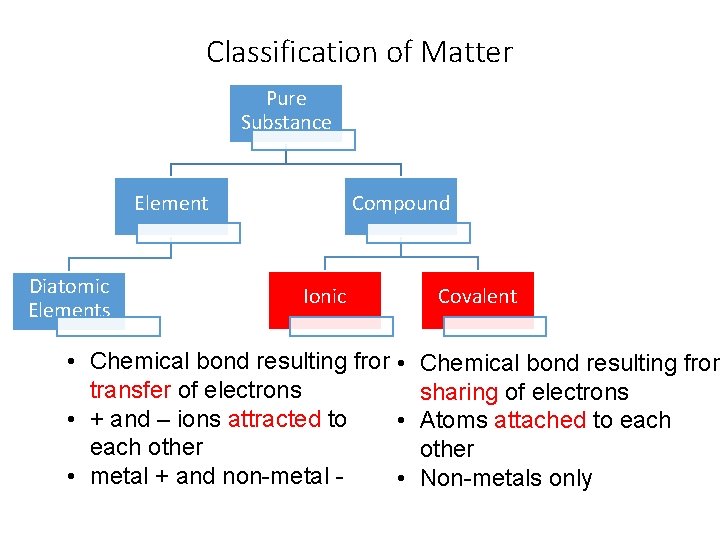
Classification of Matter Pure Substance Element Diatomic Elements Compound Ionic • Chemical bond resulting from • transfer of electrons • + and – ions attracted to • each other • metal + and non-metal • Covalent Chemical bond resulting from sharing of electrons Atoms attached to each other Non-metals only
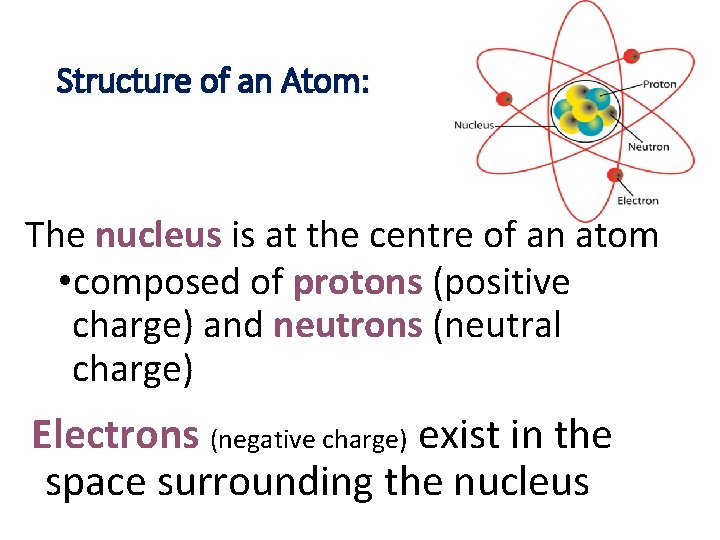
Structure of an Atom: The nucleus is at the centre of an atom • composed of protons (positive charge) and neutrons (neutral charge) Electrons (negative charge) exist in the space surrounding the nucleus
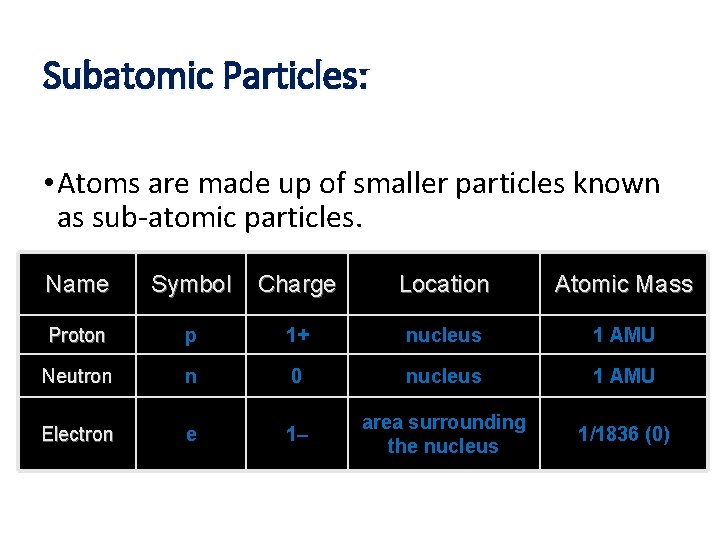
Subatomic Particles: • Atoms are made up of smaller particles known as sub-atomic particles. Name Symbol Charge Location Atomic Mass Proton p 1+ nucleus 1 AMU Neutron n 0 nucleus 1 AMU Electron e 1– area surrounding the nucleus 1/1836 (0)
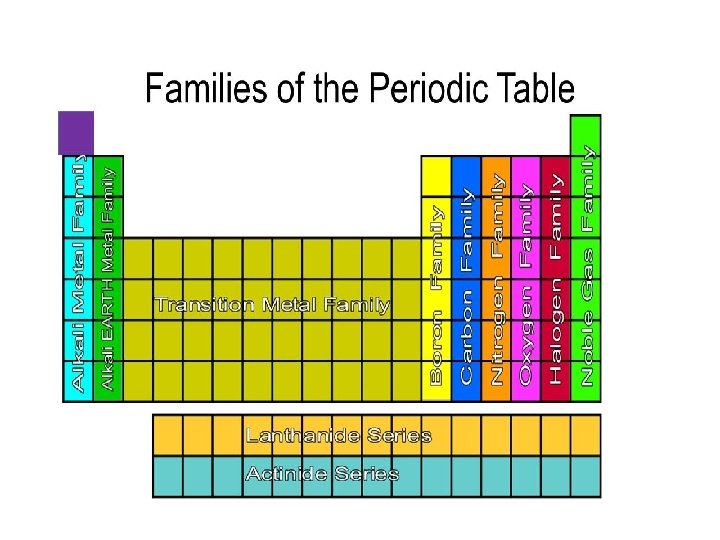
Families of the Periodic Table: • Columns of elements are called groups, or families • All elements in a family have… • similar properties • bond with other elements in similar ways • have the same number of valence electrons • Family names (on the periodic table!): • Group 1 = alkali metals • Group 2 = alkaline earth metals • Group 17 = the halogens • Group 18 = noble gases
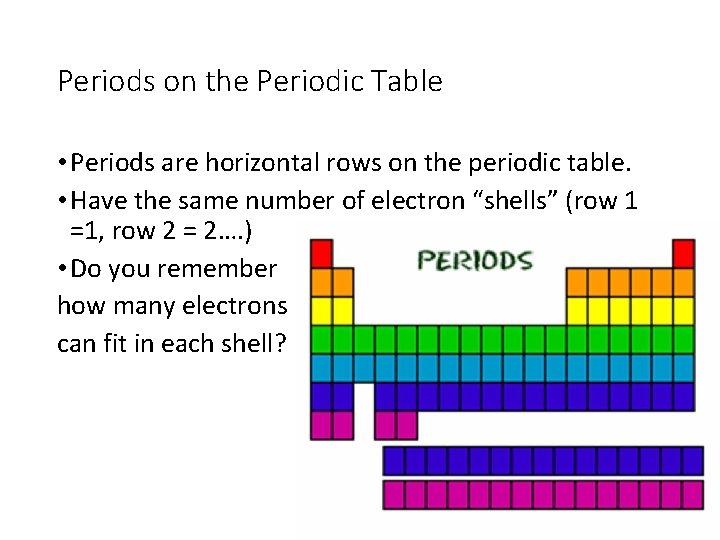
Periods on the Periodic Table • Periods are horizontal rows on the periodic table. • Have the same number of electron “shells” (row 1 =1, row 2 = 2…. ) • Do you remember how many electrons can fit in each shell?
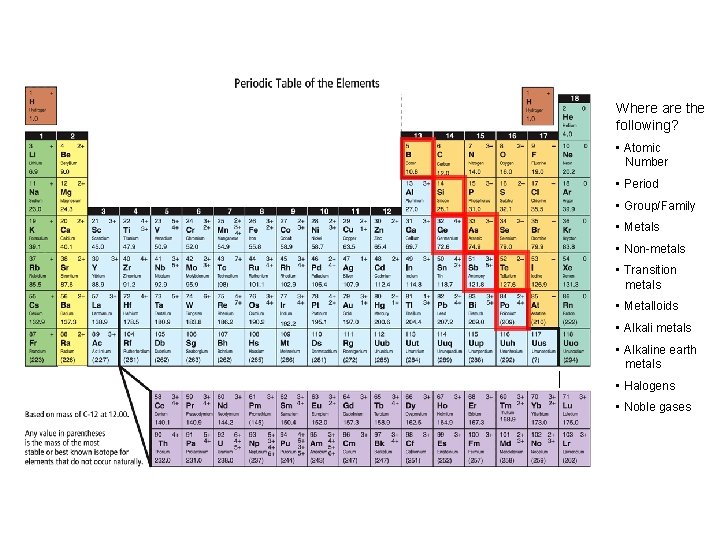
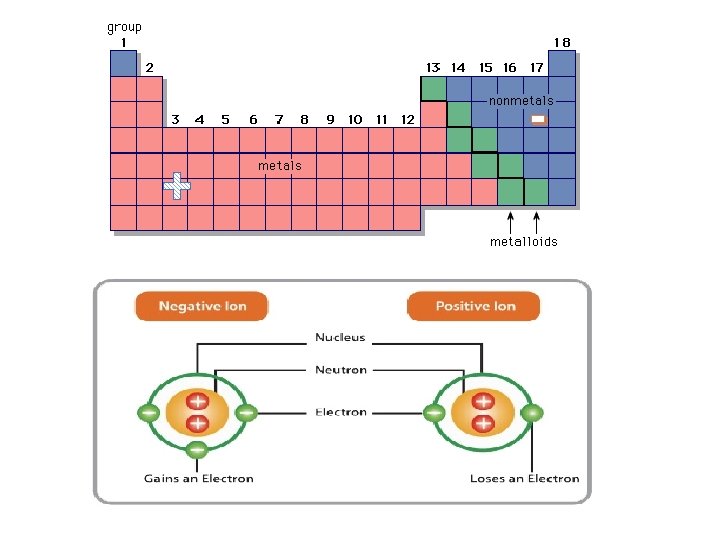
INCREASING REACTIVITY Where are the following? • Atomic Number • Period • Group/Family • Metals • Non-metals • Transition metals • Metalloids • Alkali metals • Alkaline earth metals • Halogens • Noble gases
Periodic Table & Ion Formation: • Atoms gain and lose electrons to form ions • Metals lose electrons & become positive ions (cations) • Some metals can have more than one charge (multivalent) • ie. Iron, Fe, loses either 2 (Fe 2+) or 3 (Fe 3+) electrons • Non-metals gain electrons & become negative ions (anions) • Atoms do this in an attempt to have the same number of valence electrons as the nearest noble gas – to become stable

Numbers to Remember : Atomic number = # of protons = # of electrons in atoms In ATOMS: # of protons = # of electrons in every atom AND ONLY IN ATOMS!!! (not ions) *this keeps the atom neutral since protons are (+)ive and electrons are (-)ive Positive Ions Negative Ions Metals generally form positive Ions Non-Metals generally form negative Ions They have only a few electrons in their valence so they tend to lose electrons to be “happy” They have almost a full valence so they tent to gain electrons to be “happy”
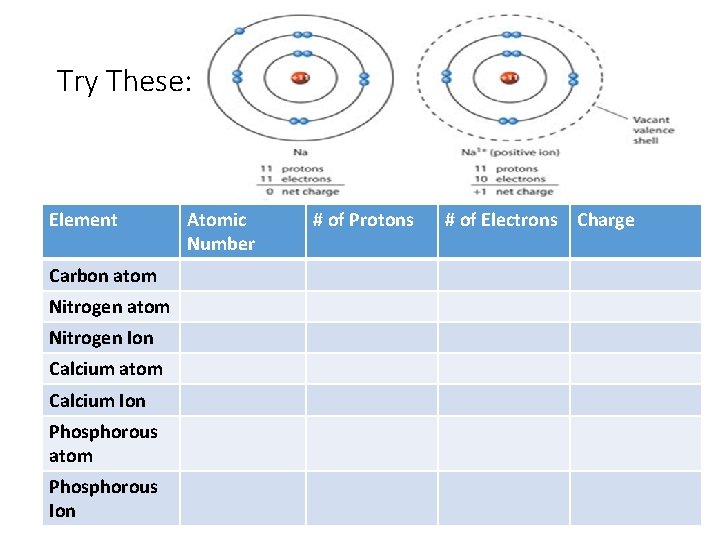
Try These: Element Carbon atom Nitrogen Ion Calcium atom Calcium Ion Phosphorous atom Phosphorous Ion Atomic Number # of Protons # of Electrons Charge
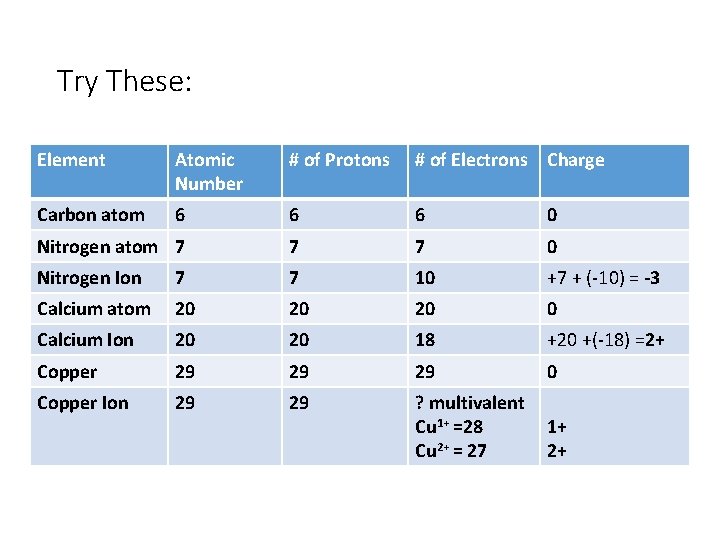
Try These: Element Atomic Number # of Protons # of Electrons Charge Carbon atom 6 6 6 0 Nitrogen atom 7 7 7 0 Nitrogen Ion 7 7 10 +7 + (-10) = -3 Calcium atom 20 20 20 0 Calcium Ion 20 20 18 +20 +(-18) =2+ Copper 29 29 29 0 Copper Ion 29 29 ? multivalent Cu 1+ =28 Cu 2+ = 27 1+ 2+

You are doing Great! Lets Keep Going Mass Number = The mass number is the ACTUAL mass of 1 atom = #Protons + # Neutrons (since both have mass) Atomic Mass = This is AVERAGE mass of the ISOTOPES of an element • Found on the periodic table and generally has a decimal. • This would be impossible in realty since the mass of an atom comes from protons and neutrons that each weight 1 amu and you can’t have part or a proton or neutron BUT it is also impossible to take a sample of an element and have only 1 atom! That same will contain many atoms and will contain a variety of the isotopes of the atoms of that element! Isotope = Same element (same atomic number / # of protons) = Different mass number because Different # of NEUTRONS
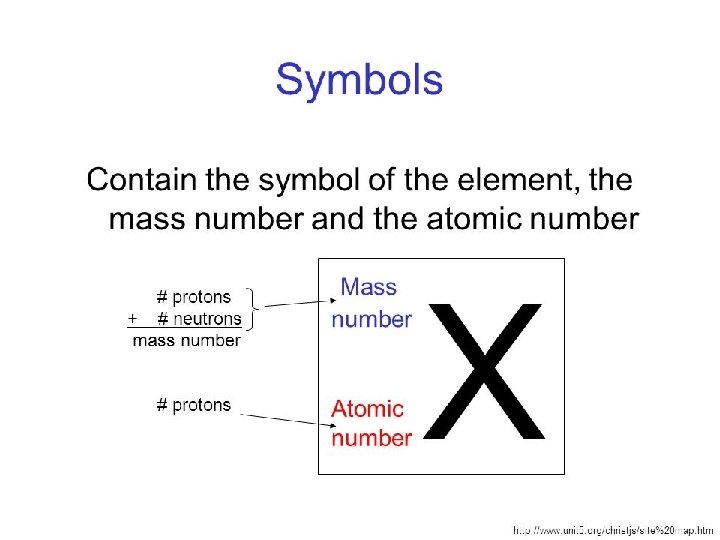
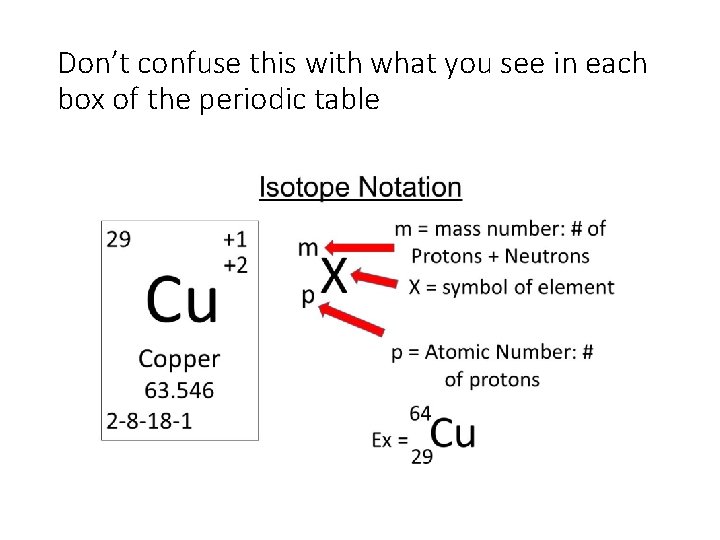
Don’t confuse this with what you see in each box of the periodic table

Practice: • Determine the number of protons neutrons and electrons for the symbol above? • If an element has 15 protons 17 neutrons and 18 electrons, write is isotope notation below?
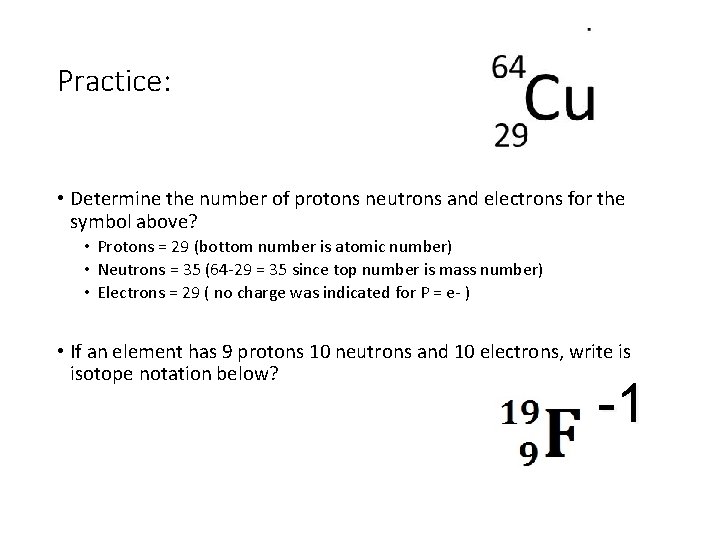
Practice: • Determine the number of protons neutrons and electrons for the symbol above? • Protons = 29 (bottom number is atomic number) • Neutrons = 35 (64 -29 = 35 since top number is mass number) • Electrons = 29 ( no charge was indicated for P = e- ) • If an element has 9 protons 10 neutrons and 10 electrons, write is isotope notation below? -1
If you are confused – Video Links Below • Difference between Mass Number and Atomic Mass • https: //www. youtube. com/watch? v=m 15 DWkk. Ge_0 • Isotopes Explained • https: //www. youtube. com/watch? v=Ebo. We. Wmh 5 Pg • Isotope Notation and Practice • https: //www. youtube. com/watch? v=BYiu 0 k. IWd 30 Otherwise Watch this before Moving on • Valence Electrons and the periodic Table • https: //www. youtube. com/watch? v=y. ADr. Wd. NTWEc

Bohr Diagrams: § Bohr diagrams show many electrons appear in each electron shell around an atom. § Each shell holds a maximum number of electrons (2, 8, 8, 18) § Electrons in the outermost shell are called valence electrons § Except for the transition elements, the last digit of the group # = # of valence electrons Note: Think of the shells as being 3 D like spheres, not 2 D like circles!
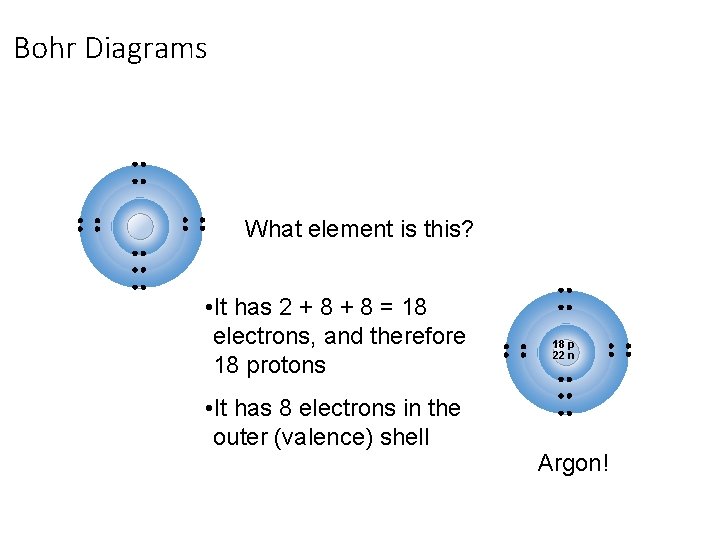
Bohr Diagrams What element is this? • It has 2 + 8 = 18 electrons, and therefore 18 protons • It has 8 electrons in the outer (valence) shell 18 p 22 n Argon!
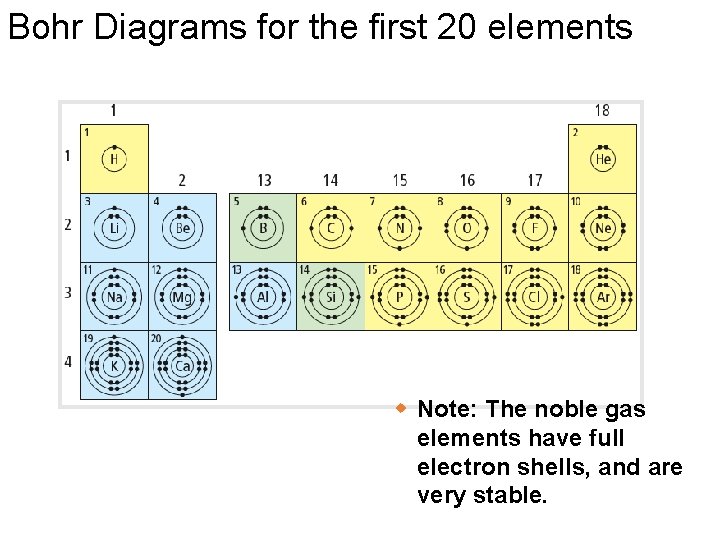
Bohr Diagrams for the first 20 elements w Note: The noble gas elements have full electron shells, and are very stable.
Annotate your Periodic Table Assignment
- Slides: 35