Atomic Theory Structure Atomic Theory Developed over 2000






- Slides: 26

Atomic Theory & Structure

Atomic Theory • Developed over 2000 years of hypothesis and observation

Democritus (460 -370 B. C. ) • If you repeatedly cut a basic material in half, you will eventually reach an indivisible particle (the atom) • Atoms – Small, hard particles made of a single material – Always moving – Form different materials by joining together • His ideas were ignored because Aristotle disagreed with him

Dalton (1766 -1844) • Demonstrated that elements combine in specific proportions because they are made of atoms • Dalton’s Atomic Theory (1803) was revised as new information was discovered

Thomson (1856 -1940) • Discovered that there are small particles inside the atom (not a solid sphere) • Plum pudding model • Chocolate chip cookie model

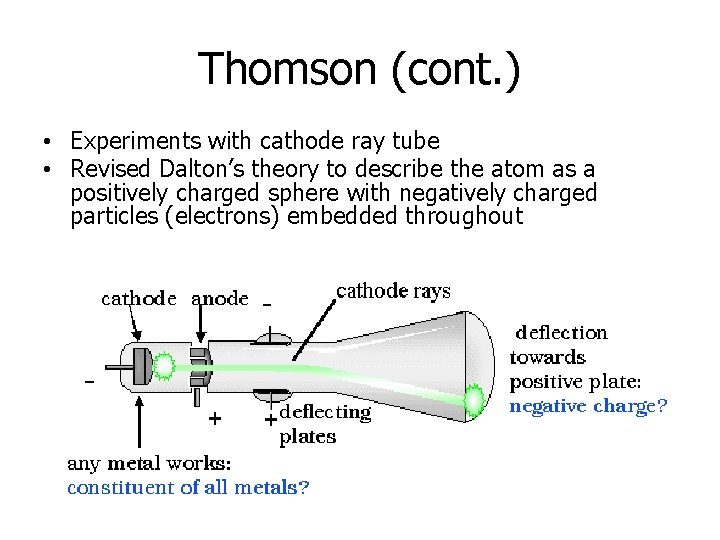
Thomson (cont. ) • Experiments with cathode ray tube • Revised Dalton’s theory to describe the atom as a positively charged sphere with negatively charged particles (electrons) embedded throughout

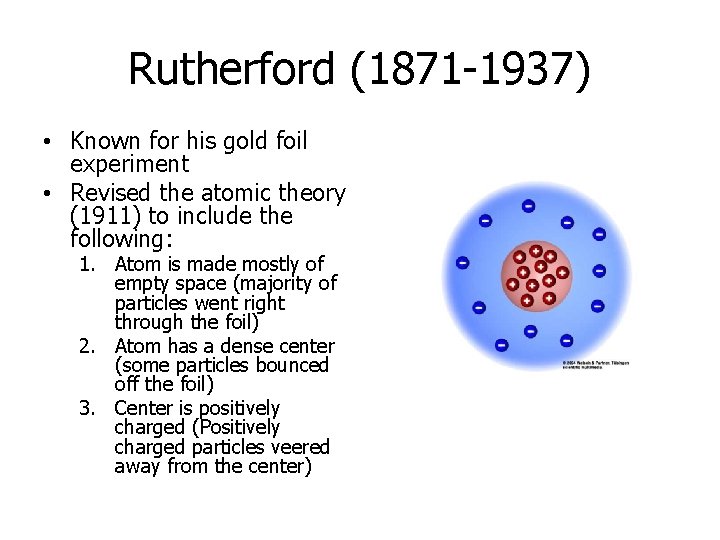
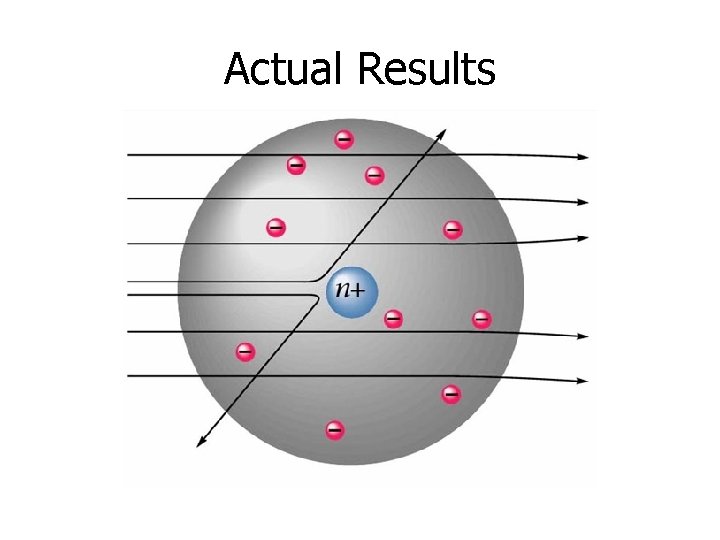
Rutherford (1871 -1937) • Known for his gold foil experiment • Revised the atomic theory (1911) to include the following: 1. Atom is made mostly of empty space (majority of particles went right through the foil) 2. Atom has a dense center (some particles bounced off the foil) 3. Center is positively charged (Positively charged particles veered away from the center)

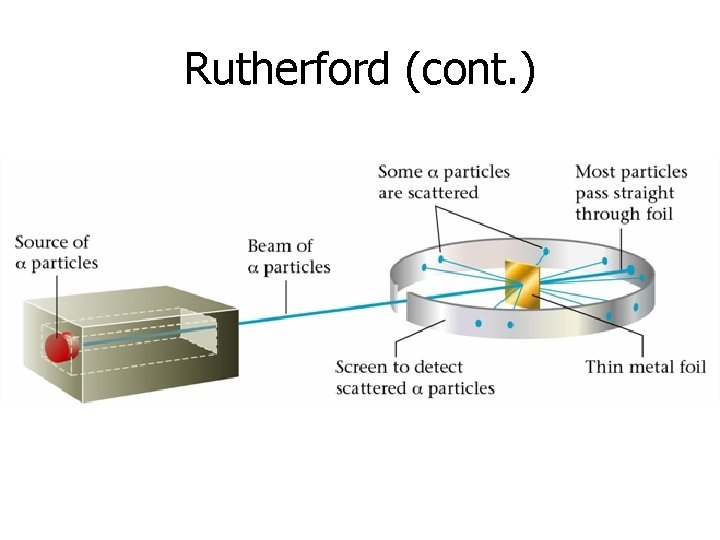
Rutherford (cont. )

Actual Results

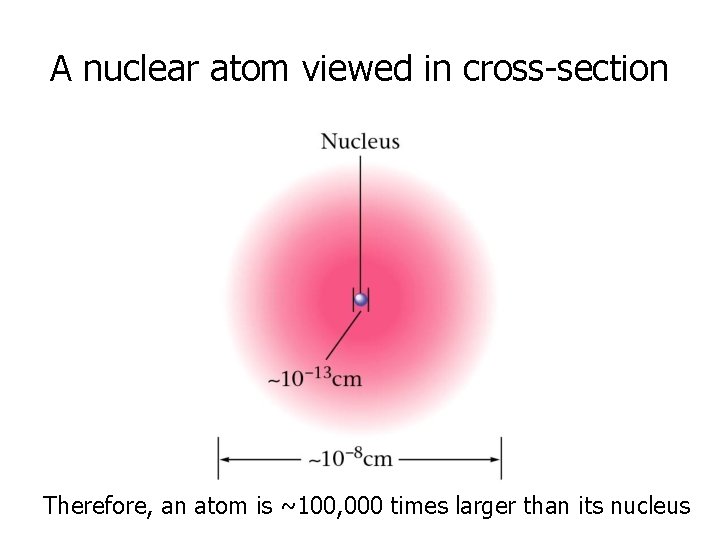
A nuclear atom viewed in cross-section Therefore, an atom is ~100, 000 times larger than its nucleus

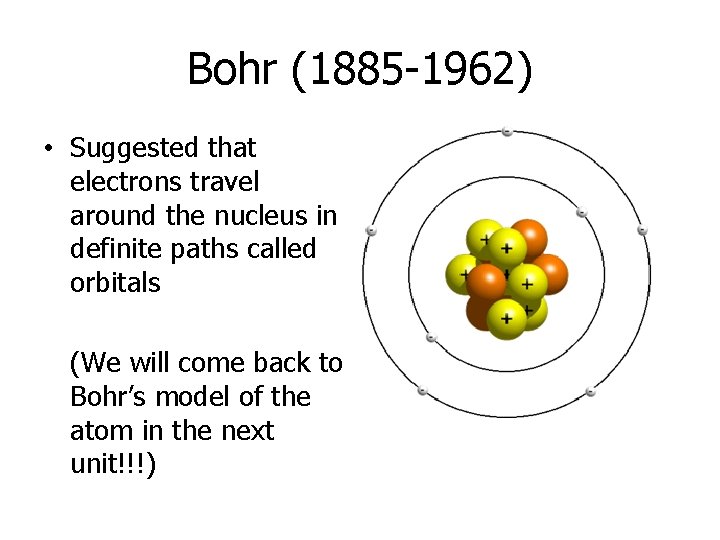
Bohr (1885 -1962) • Suggested that electrons travel around the nucleus in definite paths called orbitals (We will come back to Bohr’s model of the atom in the next unit!!!)

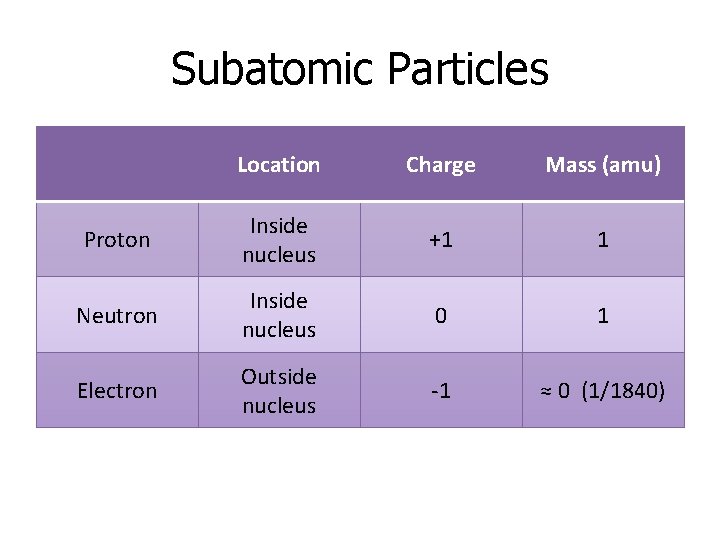
Subatomic Particles Location Charge Mass (amu) Proton Inside nucleus +1 1 Neutron Inside nucleus 0 1 Electron Outside nucleus -1 ≈ 0 (1/1840)

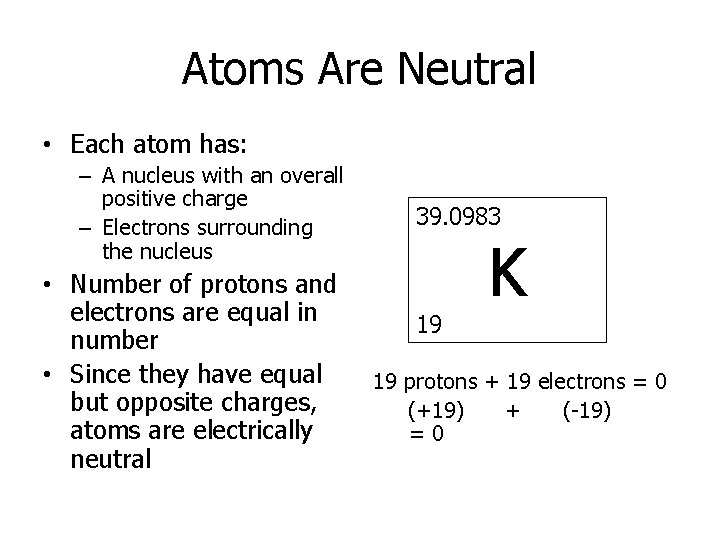
Atoms Are Neutral • Each atom has: – A nucleus with an overall positive charge – Electrons surrounding the nucleus • Number of protons and electrons are equal in number • Since they have equal but opposite charges, atoms are electrically neutral 39. 0983 19 K 19 protons + 19 electrons = 0 (+19) + (-19) =0

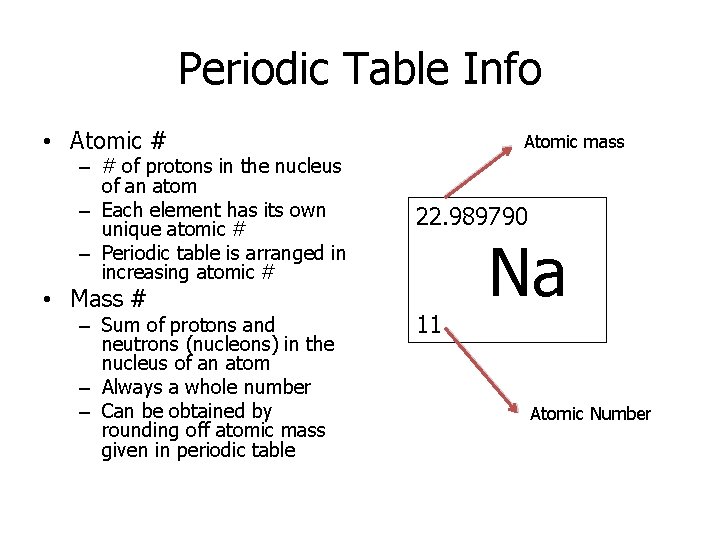
Periodic Table Info • Atomic # – # of protons in the nucleus of an atom – Each element has its own unique atomic # – Periodic table is arranged in increasing atomic # • Mass # – Sum of protons and neutrons (nucleons) in the nucleus of an atom – Always a whole number – Can be obtained by rounding off atomic mass given in periodic table Atomic mass 22. 989790 11 Na Atomic Number

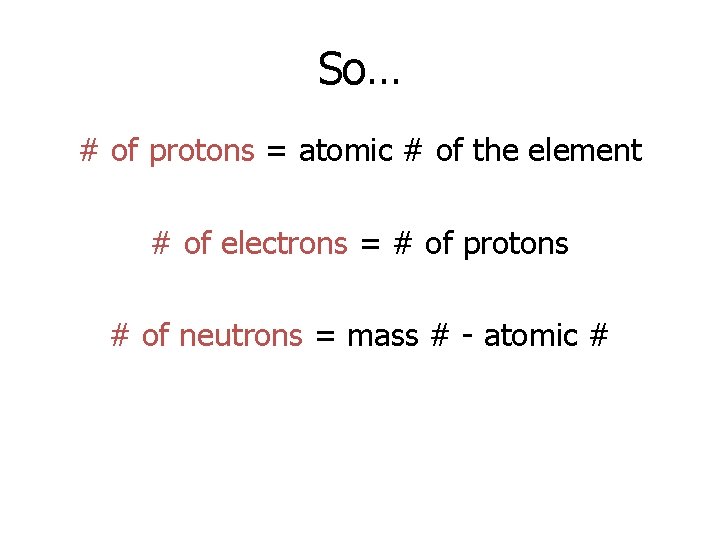
So… # of protons = atomic # of the element # of electrons = # of protons # of neutrons = mass # - atomic #

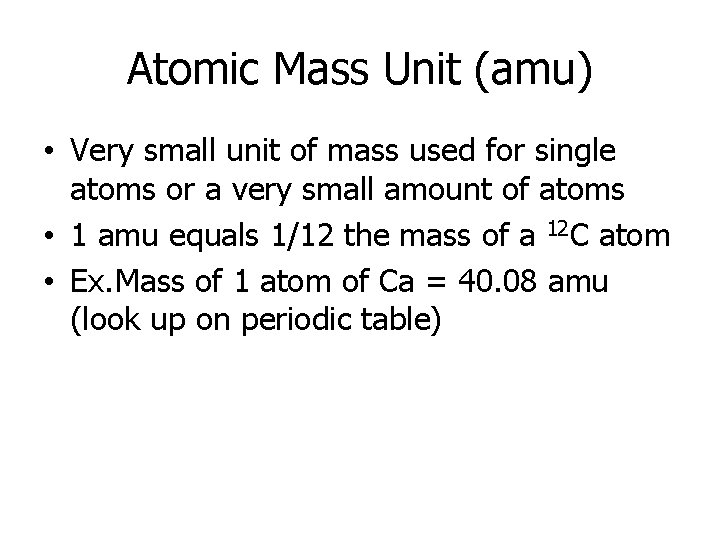
Atomic Mass Unit (amu) • Very small unit of mass used for single atoms or a very small amount of atoms • 1 amu equals 1/12 the mass of a 12 C atom • Ex. Mass of 1 atom of Ca = 40. 08 amu (look up on periodic table)

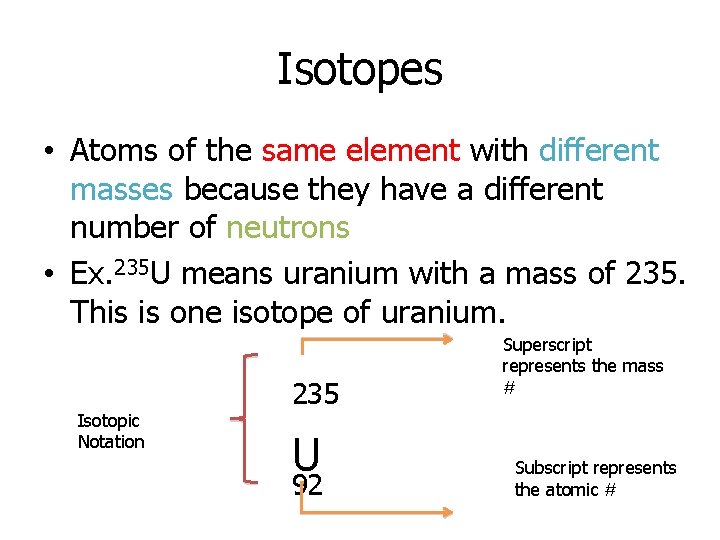
Isotopes • Atoms of the same element with different masses because they have a different number of neutrons • Ex. 235 U means uranium with a mass of 235. This is one isotope of uranium. Isotopic Notation 235 U 92 Superscript represents the mass # Subscript represents the atomic #

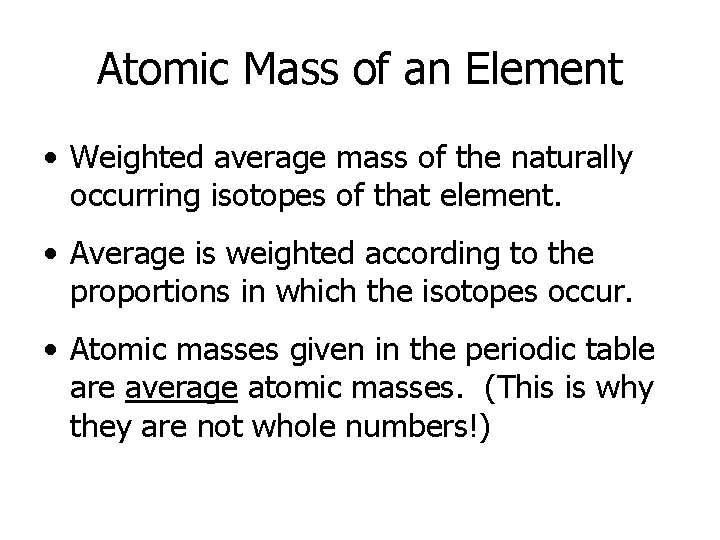
Atomic Mass of an Element • Weighted average mass of the naturally occurring isotopes of that element. • Average is weighted according to the proportions in which the isotopes occur. • Atomic masses given in the periodic table are average atomic masses. (This is why they are not whole numbers!)

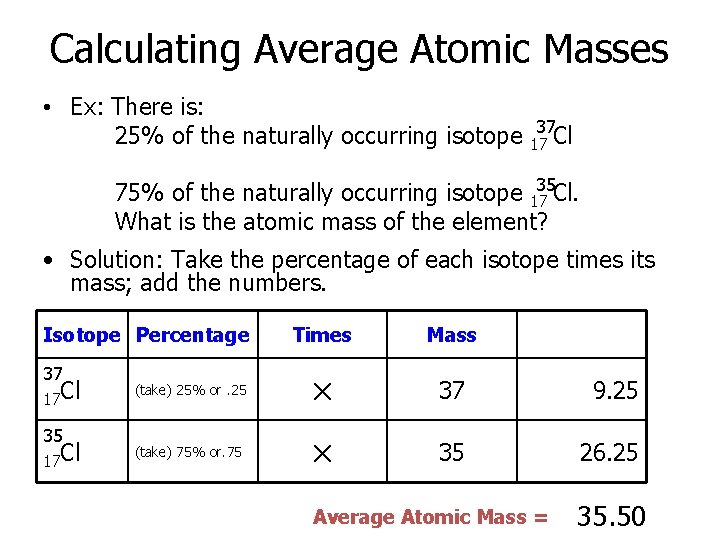
Calculating Average Atomic Masses • Ex: There is: 25% of the naturally occurring isotope 37 17 Cl 75% of the naturally occurring isotope 1735 Cl. What is the atomic mass of the element? • Solution: Take the percentage of each isotope times its mass; add the numbers. Isotope Percentage 37 17 Cl 35 17 Cl Times Mass (take) 25% or. 25 × 37 9. 25 (take) 75% or. 75 × 35 26. 25 Average Atomic Mass = 35. 50

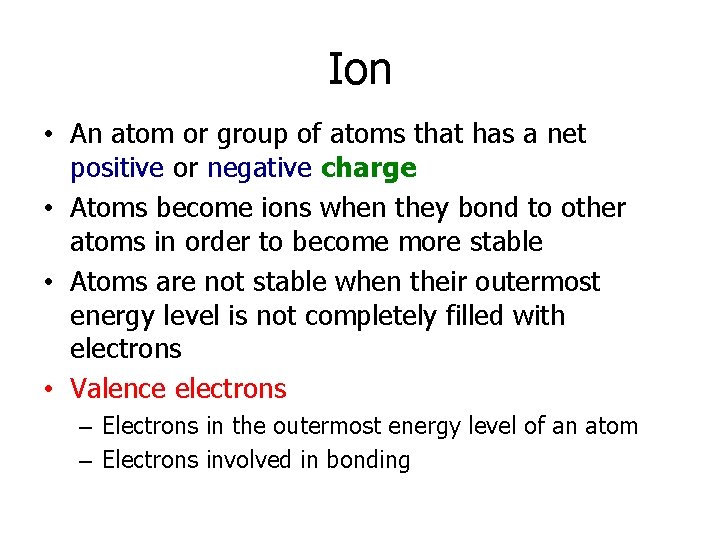
Ion • An atom or group of atoms that has a net positive or negative charge • Atoms become ions when they bond to other atoms in order to become more stable • Atoms are not stable when their outermost energy level is not completely filled with electrons • Valence electrons – Electrons in the outermost energy level of an atom – Electrons involved in bonding

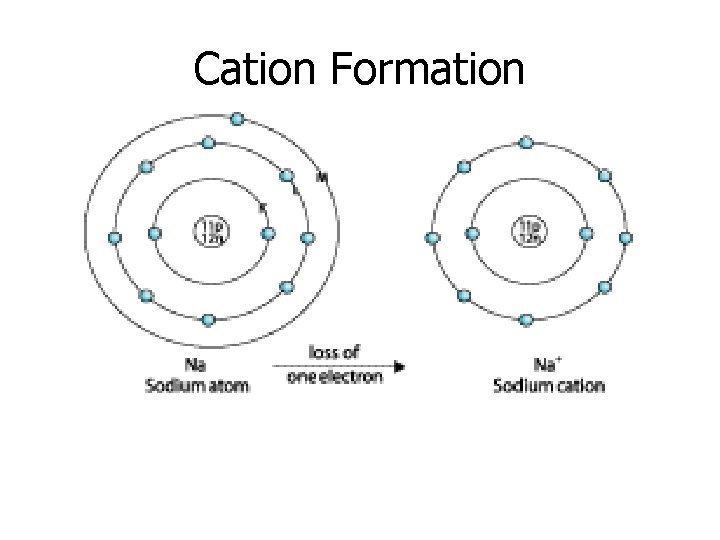
Cations • Ions with a positive charge • Metals form cations by losing an electron. • Whenever an element loses an electron, we get an ion with a +1 charge. • Metals are on the left side of the periodic table. (Metals are losers!)

Cation Formation

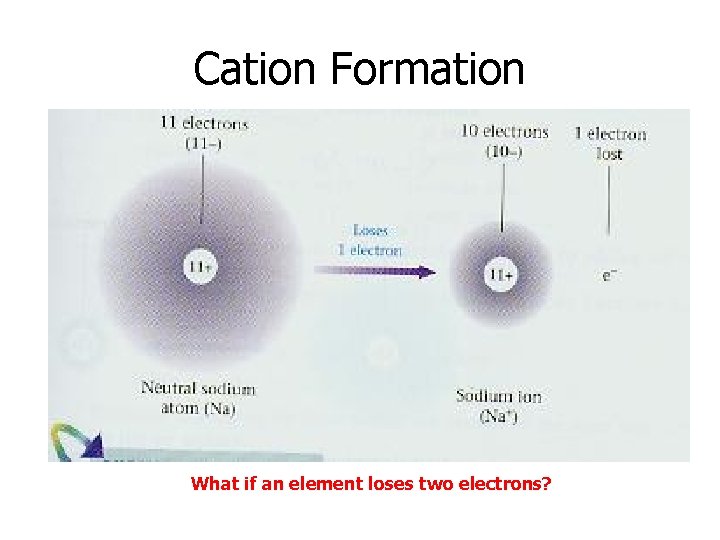
Cation Formation What if an element loses two electrons?

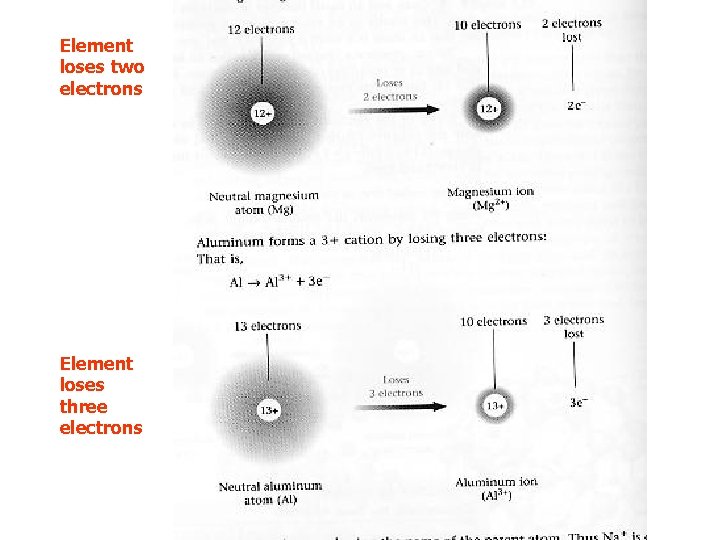
Element loses two electrons Element loses three electrons

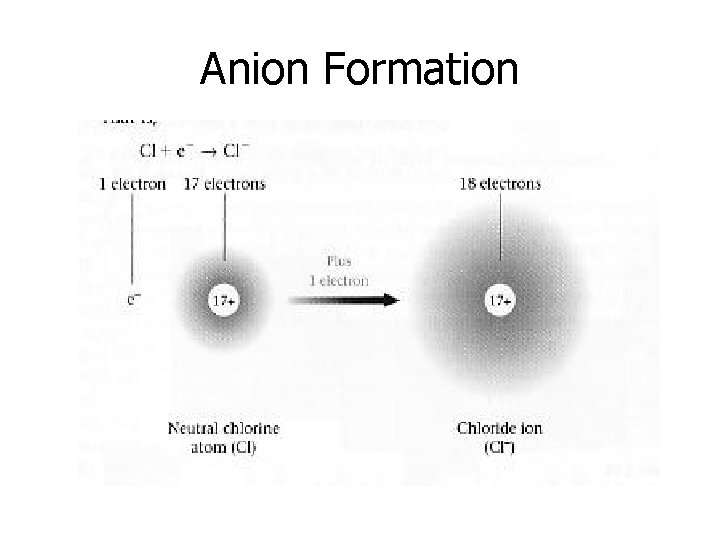
Anions • Ions with a negative charge. • Nonmetals form anions by gaining an electron. • Whenever an element gains an electron, we get an ion with a -1 charge. • Non-metals are on the right side of the periodic table. (Nonmetals are gainers!)

Anion Formation