Unit 3 Atomic Theory Structure Modern Atomic Theory

- Slides: 8

Unit 3: Atomic Theory & Structure Modern Atomic Theory

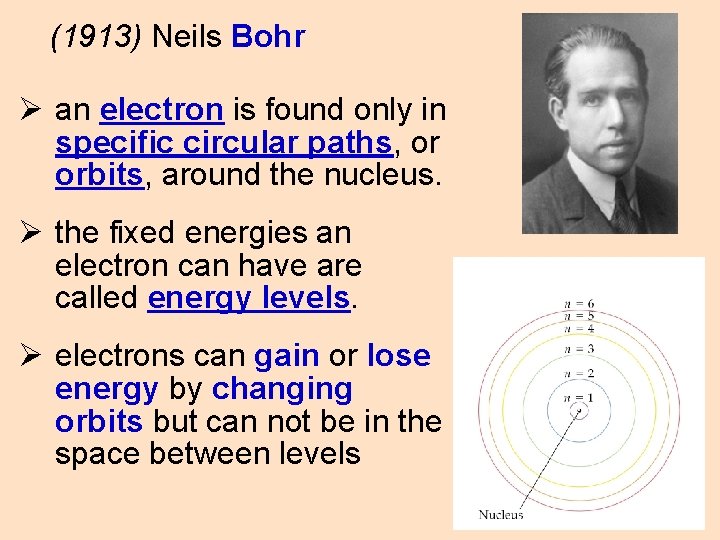
(1913) Neils Bohr Ø an electron is found only in specific circular paths, or orbits, around the nucleus. Ø the fixed energies an electron can have are called energy levels. Ø electrons can gain or lose energy by changing orbits but can not be in the space between levels

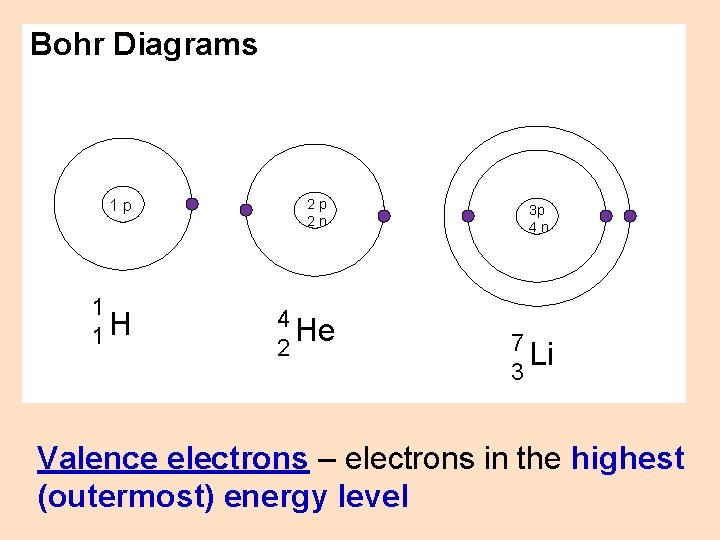
Bohr Diagrams 2, 8, 8 rule - up to 2 electrons can fit in the 1 st energy level - up to 8 electrons can fit in the 2 nd energy level - up to 8 electrons can fit in the 3 rd energy level How many energy levels? - The number of energy levels corresponds to the period number (row number) of the element Ex – H has one energy level; Li has two; Na has three, etc.

The Bohr Model Like the rungs of the strange ladder, the energy levels in an atom are not equally spaced. The higher the energy level occupied by an electron, the less energy it takes to move from that energy level to the next higher energy level.

Bohr Diagrams 1 p 1 1 H 2 p 2 n 4 He 2 3 p 4 n 7 Li 3 Valence electrons – electrons in the highest (outermost) energy level

The Electron Cloud Model (current model) • Electrons are not orbiting the nucleus in circular orbits. • Electrons are in energy levels. • Within each energy level are regions where electrons are likely to be found, called orbitals. • All orbitals together make up the electron cloud.

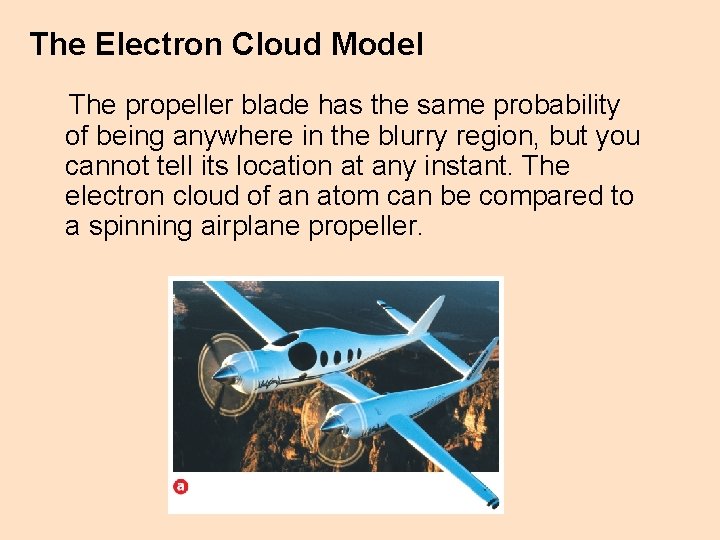
The Electron Cloud Model The propeller blade has the same probability of being anywhere in the blurry region, but you cannot tell its location at any instant. The electron cloud of an atom can be compared to a spinning airplane propeller.

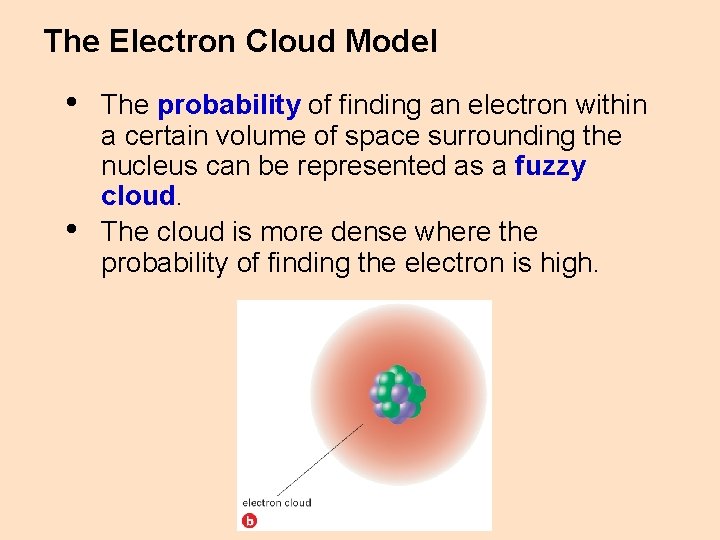
The Electron Cloud Model • • The probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. The cloud is more dense where the probability of finding the electron is high.