ATOMIC STRUCTURE ATOMIC STRUCTURE 1 2 3 4









- Slides: 27

ATOMIC STRUCTURE

ATOMIC STRUCTURE 1. 2. 3. 4. 5. Rutherford’s Experiment Basic Particles of Atomic Number and Mass Number Isotope, Isobar, Isotone, and Isoelectron Electron Configuration and Valence Electron 6. Development of Atomic Theory

1. RUTHERFORD’S EXPERIMENT CONCLUSION: v The atom consist of nucleus which has positive charge and the mass of atom is centered in the nucleus v There are many electrons which move around nucleus outside the nucleus and the amount of electrons equal to the charge of nucleus, so the atom has neutral characteristic

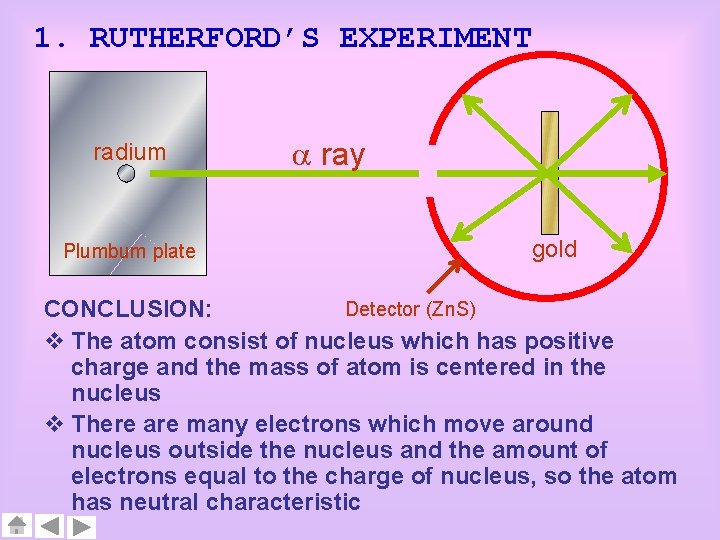
1. RUTHERFORD’S EXPERIMENT radium Plumbum plate ray gold Detector (Zn. S) CONCLUSION: v The atom consist of nucleus which has positive charge and the mass of atom is centered in the nucleus v There are many electrons which move around nucleus outside the nucleus and the amount of electrons equal to the charge of nucleus, so the atom has neutral characteristic

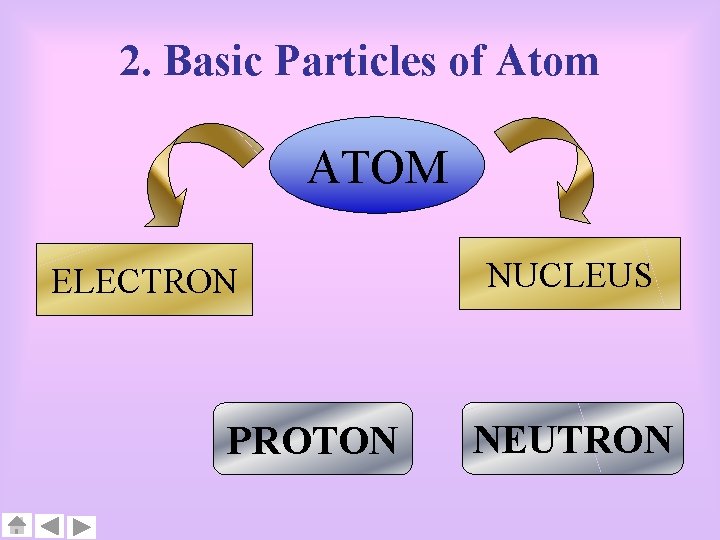
2. Basic Particles of Atom ATOM ELECTRON PROTON NUCLEUS NEUTRON

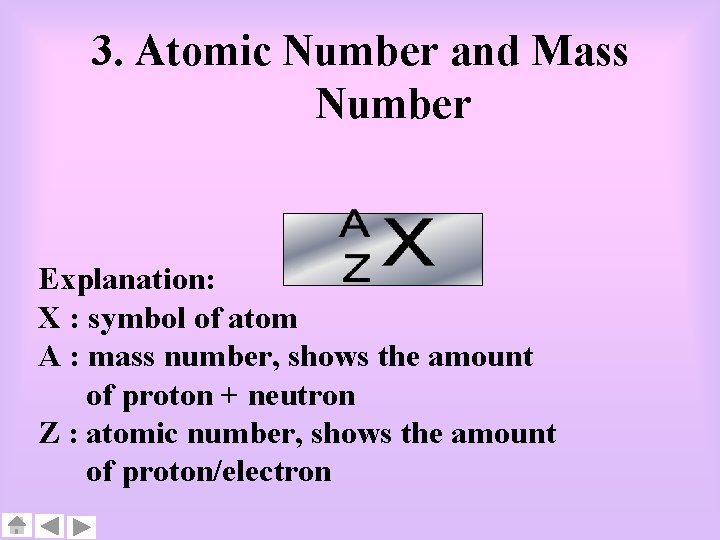
3. Atomic Number and Mass Number Explanation: X : symbol of atom A : mass number, shows the amount of proton + neutron Z : atomic number, shows the amount of proton/electron

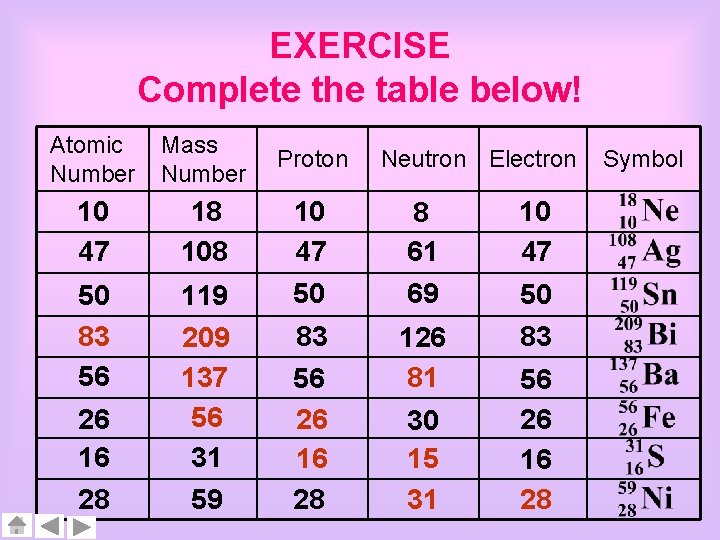
EXERCISE Complete the table below! Atomic Number Mass Number Proton 10 47 18 10 47 8 61 10 47 50 83 56 119 50 69 50 83 28 59 83 56 26 16 28 126 81 26 16 209 137 56 31 Neutron Electron 30 15 31 56 26 16 28 Symbol

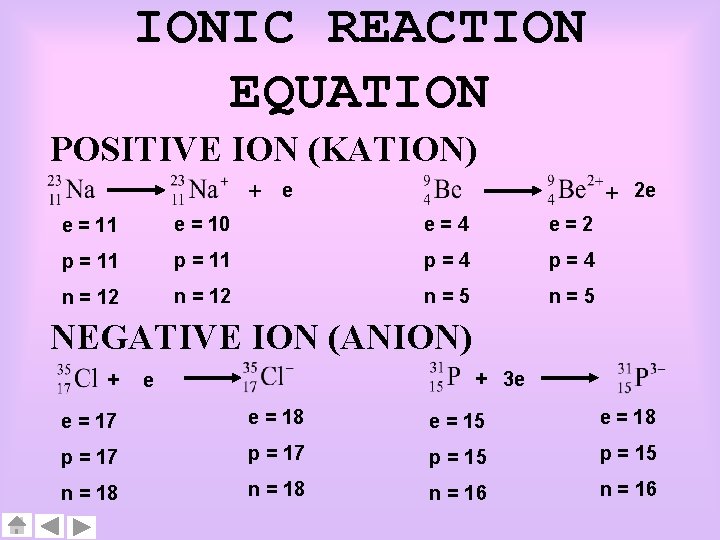
IONIC REACTION EQUATION POSITIVE ION (KATION) + e + 2 e e = 11 e = 10 e=4 e=2 p = 11 p=4 n = 12 n=5 NEGATIVE ION (ANION) + + 3 e e e = 17 e = 18 e = 15 e = 18 p = 17 p = 15 n = 18 n = 16

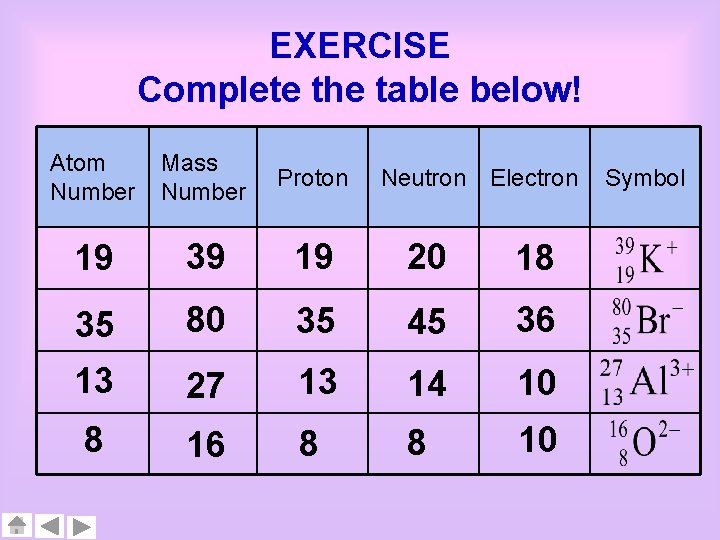
EXERCISE Complete the table below! Atom Number Mass Number Proton Neutron Electron 19 39 19 20 18 35 80 35 45 36 13 27 13 14 10 8 16 8 8 10 Symbol

4. ISOTOPE ISOBAR ISOTONE ISOELECTRON

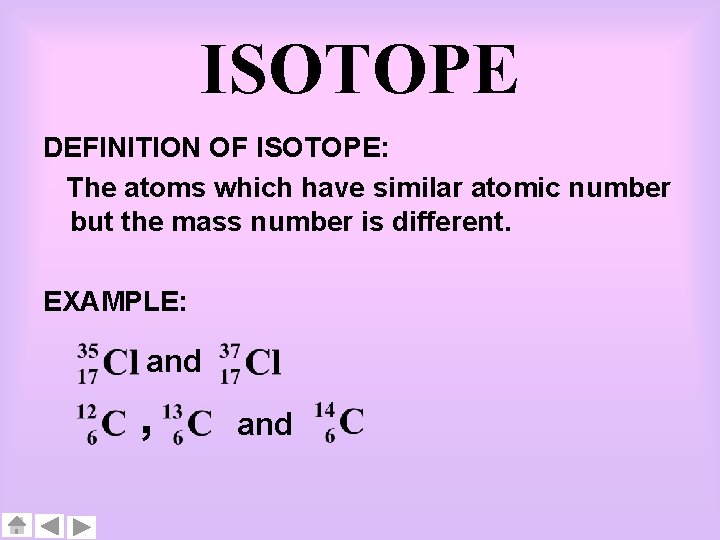
ISOTOPE DEFINITION OF ISOTOPE: The atoms which have similar atomic number but the mass number is different. EXAMPLE: and , and

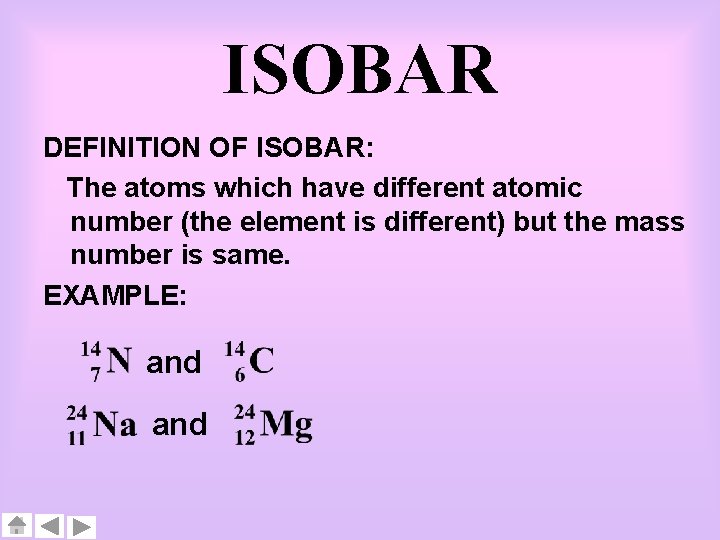
ISOBAR DEFINITION OF ISOBAR: The atoms which have different atomic number (the element is different) but the mass number is same. EXAMPLE: and

ISOTONE DEFINITION OF ISOTONE: The atoms which come from different element, but the amount of neutron is same EXAMPLE: and

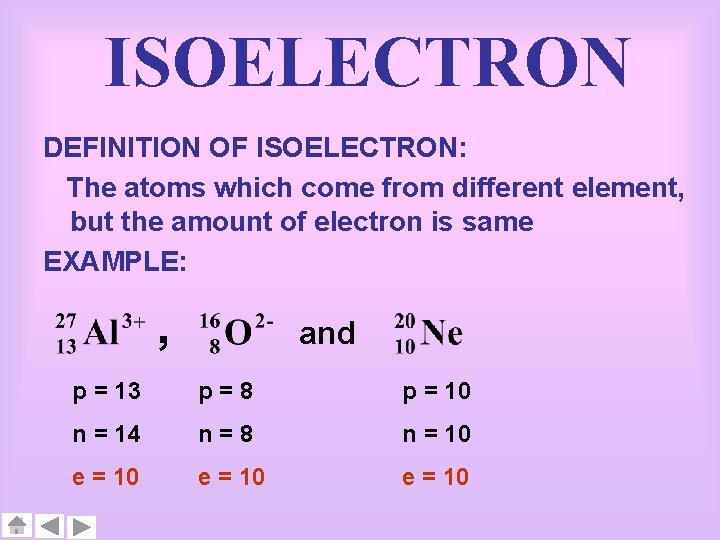
ISOELECTRON DEFINITION OF ISOELECTRON: The atoms which come from different element, but the amount of electron is same EXAMPLE: , and p = 13 p=8 p = 10 n = 14 n=8 n = 10 e = 10

5. Electron Configuration and Valence Electron

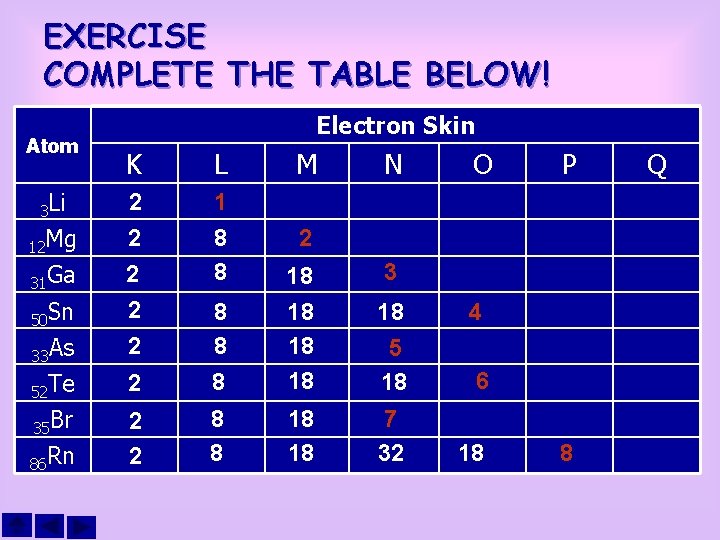
ELECTRON CONFIGURATION • • An electron configuration is the spreading of the electron in shells of an atom. The configuration must follow the regulations below: The maximum quantity of electron in the shell is 2 n 2 n Value of K shell = 1, so, the max. quantity of electron =2 n Value of L shell = 2, so, the max. quantity of electron = 8 n Value of M shell = 3, so, the max. quantity of electron =18 etc. The maximum quantity of electrons in the external shell is 8 Normally, the filling of electrons starts at the inner shell (K). Elements with atom number 1 to 18, is filled with electrons in their external shell only if the inner shell is full. The elements with atomic number more than 18, the external shell, which is the fourth shell (N) and other shell levels, can be filled with electrons, even though the third shell (M) has not full yet.

EXERCISE COMPLETE THE TABLE BELOW! Atom Electron Skin K L M 3 Li 2 12 Mg 2 2 31 Ga 50 Sn 2 2 1 8 8 33 As 2 52 Te 35 Br 86 Rn 2 8 8 8 18 18 2 2 8 8 18 18 N O P 3 18 4 5 18 6 7 32 18 8 Q

VALENCE ELECTRON 1. 2. 3. Valence electron is the quantity of electron on the external shell of which the maximum quantity is 8. Example: 11 Na Electron configuration: 2 8 1 Quantity of valence electron: 1 20 Ca Electron configuration: 2 8 8 2 Quantity of valence electron: 2 54 Xe Electron configuration: 2 8 18 18 8 Quantity of valence electron: 8

6. Development of Atomic Theory Ø Ø Ø Atomic theory of Dalton Atomic theory of J. J. Thomson Atomic theory of Rutherford Atomic theory of Niels Bohr Modern atom theory

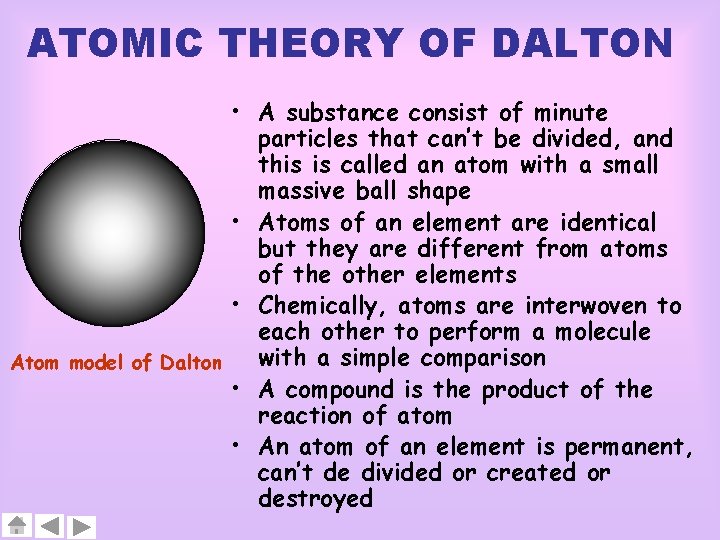
ATOMIC THEORY OF DALTON • A substance consist of minute particles that can’t be divided, and this is called an atom with a small massive ball shape • Atoms of an element are identical but they are different from atoms of the other elements • Chemically, atoms are interwoven to each other to perform a molecule with a simple comparison Atom model of Dalton • A compound is the product of the reaction of atom • An atom of an element is permanent, can’t de divided or created or destroyed

ATOM THEORY OF J. J. THOMSON Cloud of proton electron - - - Atom model of J. J. Thomson Negative charged electrons spread inside the positive charged ball (looks like the currants that spread over a piece of cake, so, its called COOKIE THEORY)

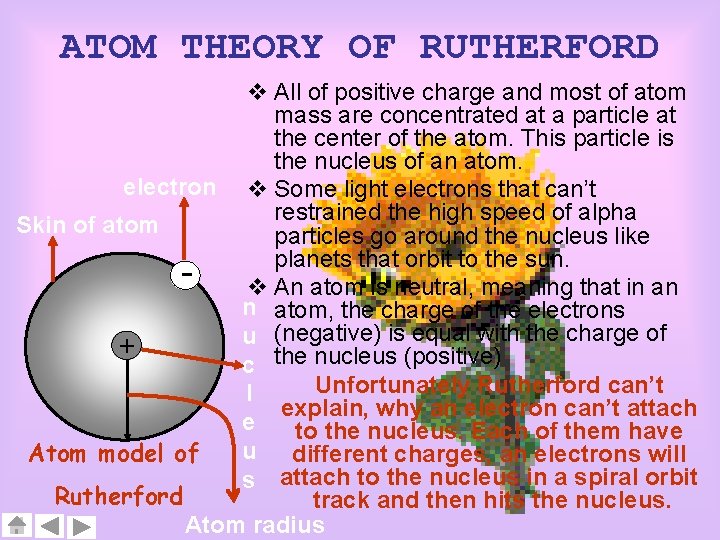
ATOM THEORY OF RUTHERFORD v All of positive charge and most of atom mass are concentrated at a particle at the center of the atom. This particle is the nucleus of an atom. electron v Some light electrons that can’t restrained the high speed of alpha Skin of atom particles go around the nucleus like planets that orbit to the sun. v An atom is neutral, meaning that in an n atom, the charge of the electrons u (negative) is equal with the charge of + c the nucleus (positive) Unfortunately Rutherford can’t l explain, why an electron can’t attach e to the nucleus. Each of them have u different charges, an electrons will Atom model of s attach to the nucleus in a spiral orbit Rutherford track and then hits the nucleus. Atom radius

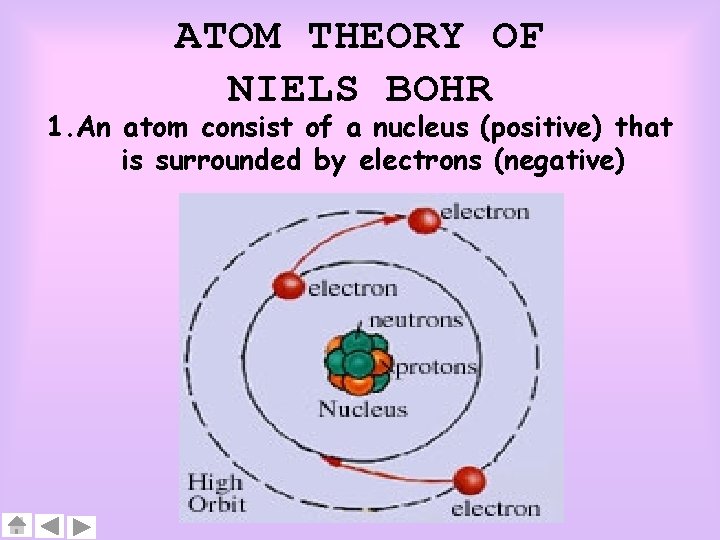
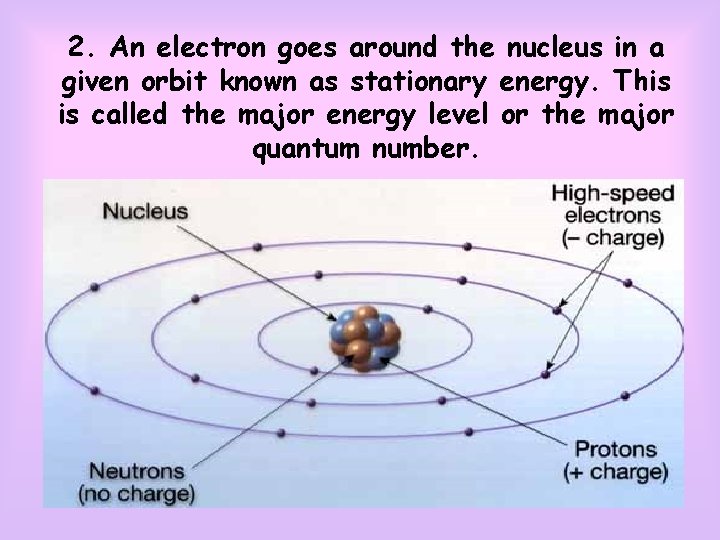
ATOM THEORY OF NIELS BOHR 1. An atom consist of a nucleus (positive) that is surrounded by electrons (negative)

2. An electron goes around the nucleus in a given orbit known as stationary energy. This is called the major energy level or the major quantum number.

3. If an electron stays on its stationary energy level, there will be no ray sparks. 4. An electron can move to the upper level if it absorbs an energy, and an electron can move to the lower level if it releases some of its energy.

THAT’S ALL

Periodic table of elements-2