Physics Chapter 4 Atomic structure atomic structure development
- Slides: 3

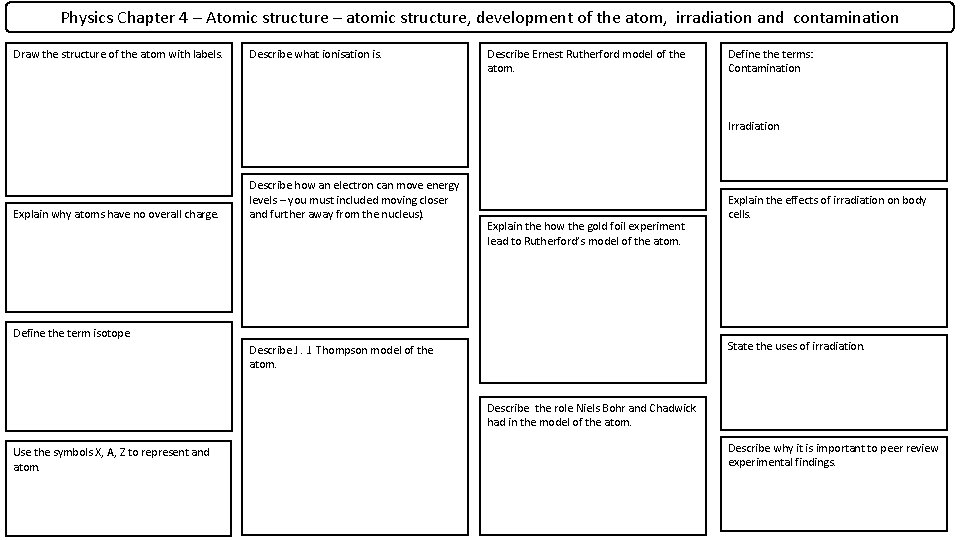
Physics Chapter 4 – Atomic structure – atomic structure, development of the atom, irradiation and contamination Draw the structure of the atom with labels. Describe what ionisation is. Describe Ernest Rutherford model of the atom. Define the terms: Contamination Irradiation Explain why atoms have no overall charge. Describe how an electron can move energy levels – you must included moving closer and further away from the nucleus). Explain the how the gold foil experiment lead to Rutherford’s model of the atom. Define the term isotope Explain the effects of irradiation on body cells. State the uses of irradiation. Describe J. J. Thompson model of the atom. Describe the role Niels Bohr and Chadwick had in the model of the atom. Use the symbols X, A, Z to represent and atom. Describe why it is important to peer review experimental findings.

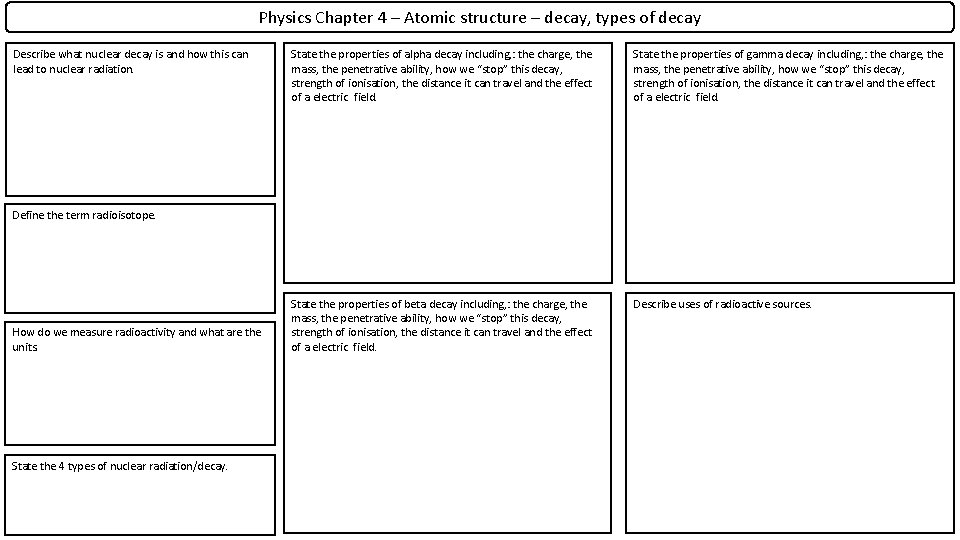
Physics Chapter 4 – Atomic structure – decay, types of decay Describe what nuclear decay is and how this can lead to nuclear radiation. State the properties of alpha decay including, : the charge, the mass, the penetrative ability, how we “stop” this decay, strength of ionisation, the distance it can travel and the effect of a electric field. State the properties of gamma decay including, : the charge, the mass, the penetrative ability, how we “stop” this decay, strength of ionisation, the distance it can travel and the effect of a electric field. State the properties of beta decay including, : the charge, the mass, the penetrative ability, how we “stop” this decay, strength of ionisation, the distance it can travel and the effect of a electric field. Describe uses of radioactive sources. Define the term radioisotope. How do we measure radioactivity and what are the units. State the 4 types of nuclear radiation/decay.

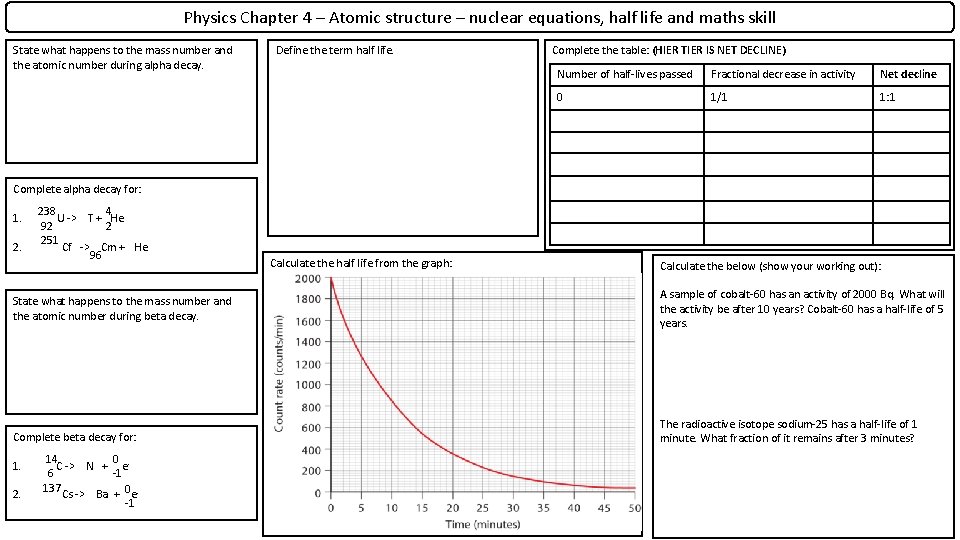
Physics Chapter 4 – Atomic structure – nuclear equations, half life and maths skill State what happens to the mass number and the atomic number during alpha decay. Define the term half life. Complete the table: (HIER TIER IS NET DECLINE) Number of half-lives passed Fractional decrease in activity Net decline 0 1/1 1: 1 Complete alpha decay for: 1. 2. 238 4 U -> T + He 92 2 251 Cf -> Cm + He 96 Calculate the half life from the graph: Calculate the below (show your working out): State what happens to the mass number and the atomic number during beta decay. A sample of cobalt-60 has an activity of 2000 Bq. What will the activity be after 10 years? Cobalt-60 has a half-life of 5 years. Complete beta decay for: The radioactive isotope sodium-25 has a half-life of 1 minute. What fraction of it remains after 3 minutes? 1. 2. 14 0 C -> N + e 6 -1 137 Cs -> Ba + 0 e-1